Monkeypox Information for Healthcare Providers
Clade I Monkeypox Virus Testing
Virus Testing
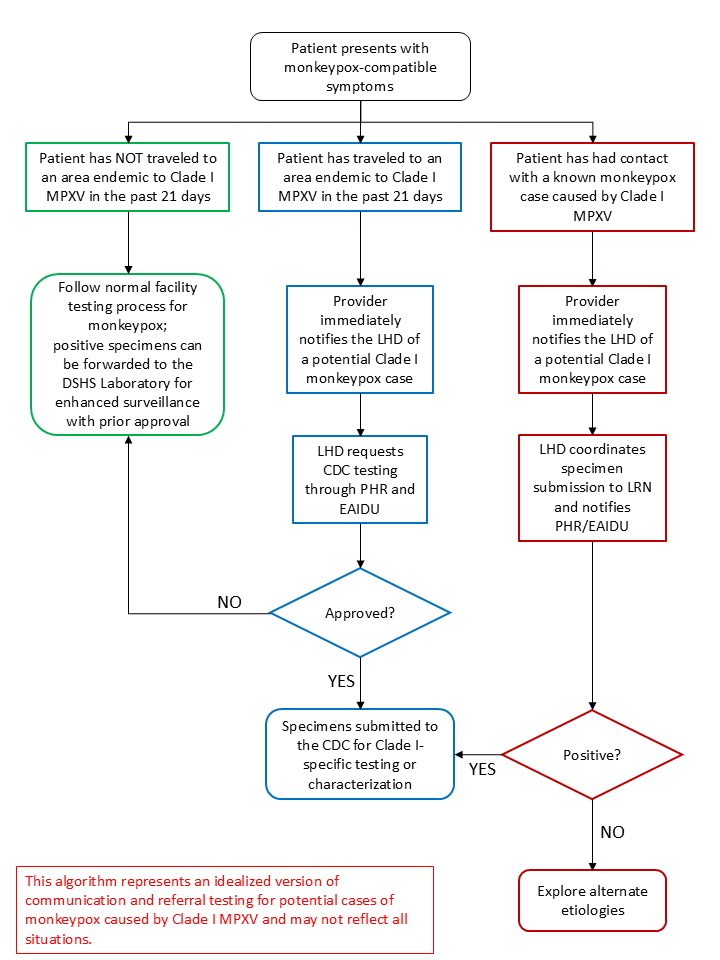
Because Clade I-specific testing is not readily available, local and regional health departments must work with clinicians in their jurisdiction to coordinate testing with DSHS and the CDC. There are two broad categories for approaching Clade I MPXV testing:
- Testing for a new suspect case of monkeypox possibly caused by Clade I MPXV (as indicated by travel history).
- Public health follow-up of close contacts of a confirmed monkeypox case caused by Clade I MPXV or retesting of a confirmed case caused by Clade I MPXV.
For patients with a travel history to an area where Clade I MPXV is endemic (e.g., the Democratic Republic of Congo, Central African Republic, Republic of the Congo, Cameroon, Gabon, and Equatorial Guinea) within 21 days of illness onset, Clade I MPXV testing should be conducted. Clinicians should immediately notify their local health department. The local health department will coordinate testing approval with the Public Health Region and DSHS. Ideally, specimens will be submitted directly from the provider for testing upon approval from DSHS and the CDC.
In the case where a case of monkeypox caused by Clade I MPXV has already been confirmed, any testing of individuals that had contact with that case and are symptomatic should be tested either at a Laboratory Response Network (LRN) facility or the CDC. As several LRN laboratories are located within Texas, results are typically available much faster and can allow for a quicker response by the local health department. As Clade I MPXV is considered a select agent, LRN laboratories are able to handle these specimens safely and in accordance with federal regulations. Most LRN laboratories, however, only provide non-variola Orthopoxvirus testing. Positive or detected non-variola Orthopoxvirus results for an individual that had close contact with a confirmed monkeypox case caused by Clade I MPXV should be presumptively considered a Clade I MPXV monkeypox case until laboratory testing provides evidence to the contrary. Specimens associated with Clade I MPXV monkeypox cases should be sent to the CDC for confirmatory testing following approval by DSHS and the CDC.
Testing Algorithm Diagram