Children
National Immunization Survey-Child (NIS-Child) 2023, Texas
Immunization Coverage among Texas Children at 24 months of age
About the National Immunization Survey-Child (NIS-Child)
The National Immunization Survey–Child (NIS-Child) is a national survey conducted annually by the Centers for Disease Control and Prevention (CDC) to assess vaccination coverage levels, or the percent of the population vaccinated, among young children.
The NIS is the only population-based survey to provide national, state, local area, and territorial estimates of vaccination coverage among children in the United States. This study collects data by administering telephone surveys to randomly selected households. To ensure the accuracy and precision of the vaccination coverage estimates, immunization data for surveyed children are also collected through a mail survey of their pediatricians, family physicians, and other health care providers. The parents and guardians of eligible children are asked during the telephone interview for consent to contact the children’s vaccination providers. Data are weighted to be representative of the population of children 24 months of age, and are adjusted for multiple phone lines, mixed telephone lines (i.e., landline and cellular), household nonresponse, and the exclusion of phoneless households. The survey also tracks progress towards the Healthy People 2030 goals.
CDC changed the way NIS results are reported starting in 2019. In the past, results were reported for children 19-35 months of age at the time of the survey. Now, results for most vaccines are reported at 24 months of age by birth year. Exceptions to this are the hepatitis B birth dose and Rotavirus vaccines which are reported at younger ages because they are completed before 24 months.
Coverage rates were reported for the U.S., Texas, and the selected areas of Bexar County and the City of Houston.
Survey Sample
2023 NIS-Child data was collected from 2021 to 2023, resulting in a statewide sample of 446 Texas children. This includes 126 children in the City of Houston and 113 children in Bexar County.
Vaccines Included
The NIS collects information on the following vaccine series to assess the percent of children that are up to date with routinely recommended vaccinations:
- Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP/DT/DTP)
- Poliovirus vaccine (Polio)
- Measles or Measles-Mumps-Rubella vaccine (MMR)
- Haemophilus influenzae type b vaccine (Hib)
- Hepatitis B vaccine (HepB)
- Varicella vaccine (Var)
- Pneumococcal conjugate vaccine (PCV)
- Hepatitis A vaccine (HepA)
- Rotavirus vaccine
- Influenza (Flu)
- 4:3:1:3*:3:1:4 series
- ≥4 doses of DTaP
- ≥3 doses of Polio
- ≥1 dose of MMR
- ≥3 doses of Hib (*3 or 4 doses depending on vaccine type)
- ≥3 doses of HepB
- ≥1 dose of Var
- ≥4 doses of PCV
The 4:3:1:3*:3:1:4 series reflects vaccine coverage for seven key vaccines combined. It measures overall alignment with the recommendations of the Advisory Committee on Immunization Practices (ACIP) for young children.
NIS Coverage Estimates
Vaccination Coverage Estimates in Texas and U.S. by 24 Monthsa of Age, by Birth Year, NIS-Child
Vaccine | U.S. Children born 2021b | Texas Children born 2020 | Texas Children born 2021b | Texas Percentage point difference |
≥4 doses diphtheria, tetanus, acellular pertussis (4+DTaP) | 79.1% | 76.3% | 83.5% | 7.2% |
≥3 doses inactivated poliovirus (3+Polio) | 91.9% | 90.2% | 93.9% | 3.7% |
≥1 dose measles, mumps, rubella (1+MMR) | 90.6% | 88.7% | 96.7%g | 8.0%h |
Haemophilus influenzae full series (Hib-FS)c | 76.1% | 78.3% | 78.2% | -0.1% |
1 dose Hepatitis B in first 3 days of life (Hep B Birth) | 78.9% | 84.3% | 82.0% | -2.3% |
≥3 doses Hepatitis B (3+HepB) | 91.1% | 89.6% | 93.0% | 3.4% |
≥1 dose varicella (1+Var) | 90.1% | 88.1% | 96.3%g | 8.2%h |
≥4 doses pneumococcal conjugate (4+PCV) | 80.8% | 78.7% | 85.7% | 7.0% |
≥ 1 dose Hepatitis A (1+HepA) | 86.2% | 89.5% | 94.1%g | 4.6% |
Rotavirus series (Rota)d | 75.4% | 73.8% | 78.9% | 5.1% |
≥2 doses influenza (2+Flu)e | 53.4% | 53.3% | 48.1% | -5.2% |
7-vaccine series (4:3:1:3c:3:1:4)f | 66.1% | 64.9% | 68.5% | 3.6% |
a Coverage estimates are at 24 months unless otherwise noted (i.e. rotavirus vaccine coverage assessed at 8 months)
b Data for the 2018 birth year are from survey years 2019, 2020 and 2021; data for the 2019 birth year are from survey years 2020, 2021 and 2022; data for the 2020 birth year are from survey years 2021, 2022, and 2023; data for the 2021 birth year are considered preliminary and come from survey years 2022 and 2023.
c Full series (FS) of either 3 or 4 doses of Hib conjugate vaccine, depending on vaccine type
d Either ≥2 or ≥3 doses of rotavirus vaccine, depending on product used
e Doses must be at least 24 days apart (four weeks, with a four-day grace period)
f 4:3:1:3:3:1:4 includes 4+ DTaP (diphtheria, tetanus, and acellular pertussis), 3+polio, 1+MMR (measles, mumps and rubella), 3 or 4 doses Hib, depending on vaccine type, 3+Hep B, 1+varicella, and 4+PCV
g Statistically significant difference (p<0.05) from the 2021 U.S. estimates
h Statistically significant difference (p<0.05) from the 2020 Texas estimate
Vaccination Coverage Estimates in Texas and U.S. by 24 Monthsa of Age, Birth Cohort, NIS-Child 2019-2023b
Vaccine | U.S. Children born 2020-2021b | Texas Children born 2018-2019 | Texas Children born 2020-2021b | Texas Percentage point difference |
≥4 doses diphtheria, tetanus, acellular pertussis (4+DTaP) | 79.3% | 78.9% | 80.0% | 1.1% |
≥3 doses inactivated poliovirus (3+Polio) | 91.9% | 92.4% | 92.1% | -0.3% |
≥1 dose measles, mumps, rubella (1+MMR) | 90.3% | 90.5% | 92.8% | 2.3% |
Haemophilus influenzae full series (Hib-FS)c | 76.8% | 77.6% | 78.3% | 0.7% |
1 dose Hepatitis B in first 3 days of life (Hep B Birth) | 79.5% | 82.4% | 83.1% | 0.7% |
≥3 doses Hepatitis B (3+HepB) | 91.1% | 89.9% | 91.3% | 1.4% |
≥1 dose varicella (1+Var) | 89.9% | 90.7% | 92.3% | 1.6% |
≥4 doses pneumococcal conjugate (4+PCV) | 80.7% | 82.8% | 82.2% | -0.6% |
≥ 1 dose Hepatitis A (1+HepA) | 86.5% | 89.9% | 91.8%g | 2.0% |
Rotavirus series (Rota)d | 75.1% | 78.0% | 76.4% | -1.6% |
≥2 doses influenza (2+Flu)e | 55.6% | 56.0% | 50.8% | -5.2% |
7-vaccine series (4:3:1:3c:3:1:4)f | 66.9% | 68.3% | 66.8% | -1.5% |
a Coverage estimates are at 24 months unless otherwise noted (i.e. rotavirus vaccine coverage assessed at 8 months)
b Data for the 2018 birth year are from survey years 2019, 2020 and 2021; data for the 2019 birth year are from survey years 2020, 2021 and 2022; data for the 2020 birth year are from survey years 2021, 2022, and 2023; data for the 2021 birth year are considered preliminary and come from survey years 2022 and 2023
c Full series (FS) of either 3 or 4 doses of Hib conjugate vaccine, depending on vaccine type
d Either ≥2 or ≥3 doses of rotavirus vaccine, depending on product used
e Doses must be at least 24 days apart (four weeks, with a four-day grace period)
f 4:3:1:3:3:1:4 includes 4+ DTaP (diphtheria, tetanus, and acellular pertussis), 3+polio, 1+MMR (measles, mumps and rubella), 3 or 4 doses Hib, depending on vaccine type, 3+Hep B, 1+varicella, and 4+PCV
g Statistically significant difference (p<0.05) than the U.S. estimates
NIS-Child Immunization Coverage Estimates in the U.S., Texas and select local areas by 24 Monthsa of Age for Children born 2020-2021b, NIS-Child 2023
Vaccine | U.S. | Texas | Bexar | City of |
≥4 doses diphtheria, tetanus, acellular pertussis (4+DTaP) | 79.3% | 80.0% | 76.9% | 73.9% |
≥3 doses inactivated poliovirus (3+Polio) | 91.9% | 92.1% | 88.2% | 90.8% |
≥1 dose measles, mumps, rubella (1+MMR) | 90.3% | 92.8% | 86.4% | 87.8% |
Haemophilus influenzae full series (Hib-FS)c | 76.8% | 78.3% | 73.4% | 76.3% |
1 dose Hepatitis B in first 3 days of life (Hep B Birth) | 79.5% | 83.1% | 73.4% | 79.7% |
≥3 doses Hepatitis B (3+HepB) | 91.1% | 91.3% | 86.0% | 89.8% |
≥1 dose varicella (1+Var) | 89.9% | 92.3% | 86.6% | 88.5% |
≥4 doses pneumococcal conjugate (4+PCV) | 80.7% | 82.2% | 75.0% | 81.3% |
≥ 1 dose Hepatitis A (1+HepA) | 86.5% | 91.8%g | 85.2% | 90.9% |
Rotavirus series (Rota)d | 75.1% | 76.4% | 73.1% | 75.4% |
≥2 doses influenza (2+Flu)e | 55.6% | 50.8% | 47.7% | 56.8% |
7-vaccine series (4:3:1:3c:3:1:4)f | 66.9% | 66.8% | 62.3% | 65.7% |
a Coverage estimates are at 24 months unless otherwise noted (i.e. rotavirus vaccine coverage assessed at 8 months)
b Data for the 2018 birth year are from survey years 2019, 2020 and 2021; data for the 2019 birth year are from survey years 2020, 2021 and 2022; data for the 2020 birth year are from survey years 2021, 2022, and 2023; data for the 2021 birth year are considered preliminary and come from survey years 2022 and 2023
c Full series (FS) of either 3 or 4 doses of Hib conjugate vaccine, depending on vaccine type
d Either ≥2 or ≥3 doses of rotavirus vaccine, depending on product used
e Doses must be at least 24 days apart (four weeks, with a four-day grace period)
f 4:3:1:3:3:1:4 includes 4+ DTaP (diphtheria, tetanus, and acellular pertussis), 3+polio, 1+MMR (measles, mumps and rubella), 3 or 4 doses Hib, depending on vaccine type, 3+Hep B, 1+varicella, and 4+PCV
g Statistically significant higher (p<0.05) than the U.S. estimates
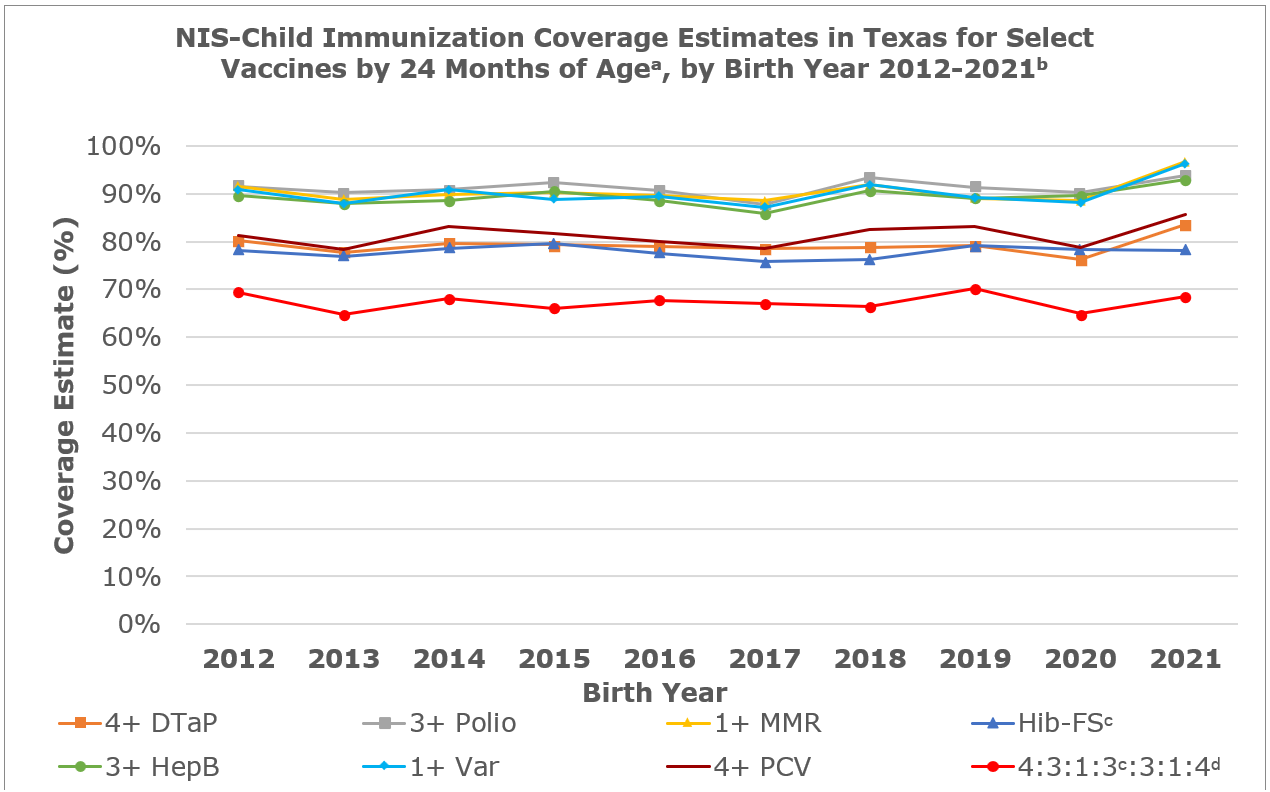
a Coverage estimates are at 24 months unless otherwise noted (i.e. rotavirus vaccine coverage assessed at 8 months)
b Data for the 2021 birth year are considered preliminary and come from survey years 2022 and 2023. Previous year data is what was reported as of
c Full series (FS) of either 3 or 4 doses of Hib conjugate vaccine, depending on vaccine type
d 4:3:1:3:3:1:4 includes 4+ DTaP (diphtheria, tetanus, and acellular pertussis), 3+polio, 1+MMR (measles, mumps and rubella), 3 or 4 doses Hib, depending on vaccine type, 3+Hep B, 1+varicella, and 4+PCV
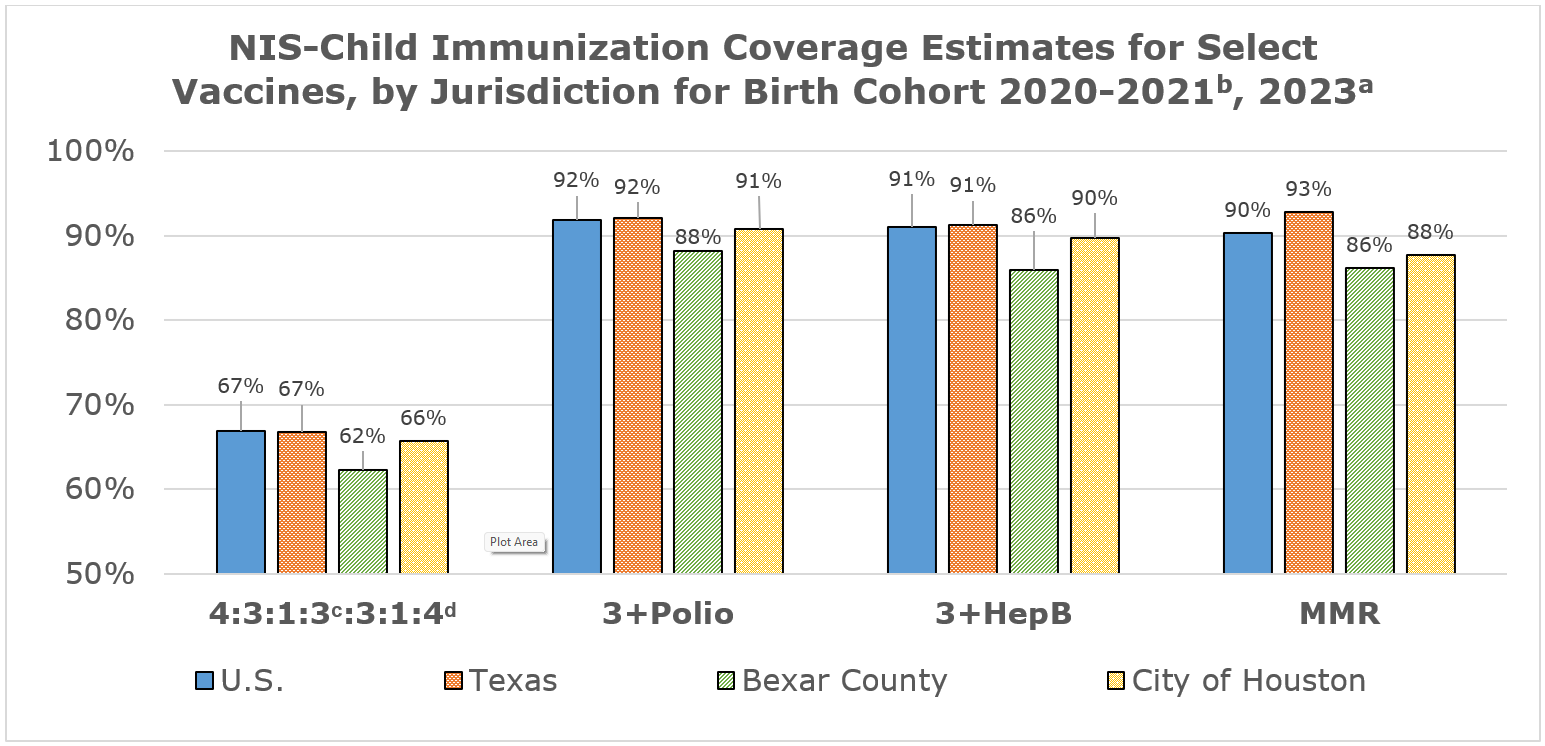
a Coverage estimates are at 24 months unless otherwise noted (i.e. rotavirus vaccine coverage assessed at 8 months)
b Data for the 2020 birth year are from survey years 2021, 2022, and 2023; data for the 2021 birth year are considered preliminary and come from survey years 2022 and 2023
c Full series (FS) of either 3 or 4 doses of Hib conjugate vaccine, depending on vaccine type
d4:3:1:3:3:1:4 includes 4+ DTaP (diphtheria, tetanus, and acellular pertussis), 3+polio, 1+MMR (measles, mumps and rubella), 3 or 4 doses Hib, depending on vaccine type, 3+Hep B, 1+varicella, and 4+PCV