Microbiology Unit Notices
DSHS Laboratory Services Section – Notice to Public
DSHS Austin Microbiology Laboratory Closed
Presidents Day, February 16
The DSHS Austin Microbiology Laboratory will be closed Monday, February 16 in observance of Presidents Day.
- Do not ship microbiology specimens for Monday, February 16 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8 a.m. Monday, February 16.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, February 13 from the Rabies Laboratory.
- TB Elimination submitters should not collect or ship specimens on Friday, February 13.
- Do not collect or ship gonorrhea antimicrobial susceptibility (GC AST) specimens Sunday, February 15 as these specimens must be tested within 24 hours of collection.
- As is standard practice, please do not collect or ship GC AST specimens on Friday for Saturday delivery.
The Microbiology Laboratory will resume normal business hours Tuesday, February 17.
Questions about Shipping and the Holiday Closure?
Specimen Acquisition Team: 512-776-7598
Rabies Team: 512-776-7595; rabies.team@dshs.texas.gov
TB Team: 512-776-7342; mycobacteriology@dshs.texas.gov
Posted February 11, 2026
New World Screwworm Specimen Handling Requirements: Collection, Shipping and Disposal
On January 20, 2026, the Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN) Health Advisory to notify public health partners about recent New World screwworm (NWS) cases in northern Mexico, and to increase awareness and preparedness for its potential geographic spread into the United States.
New World screwworm (NWS) is a painful parasitic infestation caused by the larvae of Cochliomyia hominivorax. The flies lay eggs in a wound on a human or other warm-blooded animal, where the larvae hatch and feed on tissue. NWS has the potential to cause severe illness and to harm agricultural production in Texas if it is introduced. It is imperative that suspected NWS larvae from human cases are sent to a public health laboratory for identification; this is the only way a case can be confirmed.
Specimen Collection Recommendations
NWS infestations in humans are treated by removing ALL larvae from the wound site. In some cases, surgical removal may be necessary since larvae can burrow deep into wounds to feed on live tissue.
- All extracted larvae should be placed immediately into 70% ethanol or isopropanol in a leak proof container that holds enough liquid to submerge each larva.
- After extraction of the larvae, remove 10 larvae from the alcohol container and place into a second vial with 70% ethanol or isopropanol for laboratory identification.
- If there are fewer than 10 larvae collected, submit them all.
- The larvae submitted for identification should be of varying sizes and collected from different depths within the wound.
Specimen Shipping Recommendations
- Healthcare providers and laboratories should immediately report suspected NWS cases in humans to their local health department to coordinate shipping specimens to their local public health laboratory.
- The local health department will work with the Texas Department of State Health Services (DSHS) Laboratory in Austin for sample shipment and identification.
- Only specimens obtained from humans are accepted.
- The DSHS Laboratory does not accept NWS specimens from the public.
- Refer to the DSHS Laboratory’s NWS specimen submission job aid: New World Screwworm Job Aid
Specimen Disposal
- Submerge all remaining larvae and eggs in a separate leak-proof container in 70% ethanol or isopropanol and place the container into a zip-top plastic bag and seal it.
- Dispose of the sealed bag according to your facility’s medical waste disposal protocol.
- Do not dispose of any larvae in the trash or outside in the ground.
- Failure to kill and properly dispose of all NWS larvae or eggs may result in the introduction and spread of NWS in the local environment.
New World Screwworm Testing, Collection, or Specimen Submission Questions?
- NWS Case Reporting: Local Health Department Contact List
- Call the DSHS Medical Parasitology Lab: 512-776-7560
Email: Medical.Parasitology@dshs.texas.gov - Submitter ID Numbers or Submission Forms: 512-776-7578 or LabInfo@dshs.texas.gov
- Overnight Shipping Address: Salvador Arreola, Texas Dept. of State Health Services, Public Health Laboratory Division, 1100 W. 49th Street, Austin, TX 78756-3199
For more details on NWS, a CDC health advisory can be found here: New World Screwworm: Outbreak Moves into Northern Mexico | HAN | CDC
New World Screwworm Specimen Handling Requirements: Collection, Shipping and Disposal
On January 22, 2026, the Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN) Health Advisory to notify public health partners about recent New World screwworm (NWS) cases in northern Mexico, and to increase awareness and preparedness for its potential geographic spread into the United States.
New World screwworm (NWS) is a painful parasitic infestation caused by the larvae of Cochliomyia hominivorax. The flies lay eggs in a wound on a human or other warm-blooded animal, where the larvae hatch and feed on tissue. NWS has the potential to cause severe illness and to harm agricultural production in Texas if it is introduced. It is imperative that suspect NWS larvae from human cases are sent to a public health laboratory for identification; this is the only way a case can be confirmed.
Specimen Collection Recommendations
NWS infestations in humans are treated by removing ALL larvae from the wound site. In some cases, surgical removal may be necessary since larvae can burrow deep into wounds to feed on live tissue.
- All extracted larvae should be placed immediately into 70% ethanol or isopropanol in a leak proof container that holds enough liquid to submerge each larva.
- After extraction of the larvae, remove 10 larvae from the alcohol container and place into a second vial with 70% ethanol or isopropanol for laboratory identification.
- If there are fewer than 10 larvae collected, submit them all.
- The larvae submitted for identification should be of varying sizes and collected from different depths within the wound.
Specimen Shipping Recommendations
- Healthcare providers and laboratories should immediately report suspected NWS cases in humans to their local health department to coordinate shipping specimens to their local public health laboratory.
- The local health department will work with the Texas Department of State Health Services (DSHS) Laboratory in Austin for sample shipment and identification.
- Only specimens obtained from humans are accepted.
- The DSHS Laboratory does not accept NWS specimens from the public.
- Refer to the DSHS Laboratory’s NWS specimen submission job aid: New World Screwworm Job Aid
Specimen Disposal
- Submerge all remaining larvae and eggs in a separate leak-proof container in 70% ethanol or isopropanol and place the container into a zip-top plastic bag and seal it.
- Dispose of the sealed bag according to your facility’s medical waste disposal protocol.
- Do not dispose of any larvae in the trash or outside in the ground.
- Failure to kill and properly dispose of all NWS larvae or eggs may result in the introduction and spread of NWS in the local environment.
New World Screwworm Testing, Collection, or Specimen Submission Questions?
- NWS Case Reporting: Local Health Department Contact List
- Call the DSHS Medical Parasitology Lab: 512-776-7560
- Email: Medical.Parasitology@dshs.texas.gov
- Submitter ID Numbers or Submission Forms: 512-776-7578 or LabInfo@dshs.texas.gov
- Overnight Shipping Address: Salvador Arreola, Texas Dept. of State Health Services, Public Health Laboratory Division, 1100 W. 49th Street, Austin, TX 78756-3199
For more details on NWS, a CDC health advisory can be found here: New World Screwworm: Outbreak Moves into Northern Mexico | HAN | CDC,
The DSHS health advisory can be found here: DSHS Health Advisory: Raising Awareness about New World Screwworm | Texas DSHS
Posted February 05, 2026
Updated CPT and Procedure Code for Gonorrhea/Chlamydia (GC/CT), Amplified RNA Probe Test
The CPT and procedure codes for the Gonorrhea/Chlamydia (GC/CT), Amplified RNA Probe test have changed, effective 1/1/2026. The updated Austin Laboratory Fee Schedule reflects:
- The new CPT code is 87494. Previously, the test had two CPT codes: 87491 and 87591.
- The DSHS Laboratory Procedure code changed from MBB0191A to MBB0191B.
The fee charged to submitters is not impacted. The Austin Laboratory Fee Schedule is available on the DSHS website: https://www.dshs.texas.gov/laboratory-services/laboratory-fee-schedule
Questions?
Please contact LabAccounting@dshs.texas.gov.
Posted February 03, 2026
DSHS Austin Laboratory Delayed Opening
January 27, 2026
The DSHS Laboratory in Austin, TX, will open at 12:00 PM on Tuesday, January 27, 2026, due to inclement weather and hazardous road conditions.
- We understand that some areas may still be negatively affected by cold and icy conditions so please check with your courier for local service alerts. Courier services may experience delays due to prolonged cold or hazardous conditions.
- Received specimens will be tested as soon as possible, and backlogs will be resolved during business hours.
- Please Note: Specimens received outside of their acceptability window will be unsatisfactory for testing.
The DSHS Austin Laboratory appreciates the dedication and commitment of healthcare providers to keep Texans healthy.
Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Posted January 26, 2026
DSHS Austin Laboratory Inclement Weather Closure
January 26, 2026
The DSHS Laboratory in Austin, TX, will be closed on Monday, January 26, 2026, due to inclement weather and hazardous road conditions. The closure may cause testing delays.
- Continue to ship all specimens per regular guidelines.
- Due to the potential for shipping delays, please transfer serum for glucose and lipid testing from SST tubes into new tubes and freeze them. Then ship them only after weather conditions improve.
- Courier services may experience delays due to the weather.
- Please check with your courier for local service alerts.
- All specimens will be processed as soon as possible after resuming normal operations.
- Note that specimens received outside of their acceptability window will be unsatisfactory for testing.
- The Laboratory will work at maximum capacity to resolve any specimen backlog.
The DSHS Austin Laboratory appreciates the dedication and commitment of healthcare providers to keep Texans healthy.
Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Posted January 25, 2026
DSHS Austin Microbiology Laboratory Closed on
Martin Luther King, Jr. Day
The DSHS Austin Microbiology Laboratory will be closed Monday, January 19 in observance of Martin Luther King, Jr. Day.
- Do not ship microbiology specimens for Monday, January 19 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8 a.m. Monday, January 19.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, January 16 from the Rabies Laboratory.
- Do not collect or ship gonorrhea antimicrobial susceptibility (GC AST) specimens Sunday, January 18 as these specimens must be tested within 24 hours of collection.
- As is standard practice, please do not collect or ship GC AST specimens on Friday for Saturday delivery.
The Microbiology Laboratory will resume normal business hours Tuesday, January 20.
Questions about Shipping and the Holiday Closure?
Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
Specimen Receiving Team: 512-776-7598 or 1-888-963-7111 ext. 7598 (toll free)
Posted January 14, 2026
DSHS Microbiology Laboratory Christmas 2025
and New Year 2026 Closure Reminder
Except for the Rabies Lab, the DSHS Microbiology Laboratory will be closed, and no testing will occur Wednesday, 12/24/2025 through Saturday, 12/27/2025. The DSHS Laboratory is closed on Sundays.
Testing services will resume as normal, Monday, 12/29/2025.
- The Laboratory will be closed on New Year’s Day, Thursday 01/01/2026 and will reopen Friday 01/02/2026.
Submitters are responsible for the timely and appropriate shipping of specimens to the Laboratory.
See below for details on selected testing.
Rabies Specimens
- Do not ship specimens for delivery between Wednesday, 12/24/2025 and Friday, 12/26/2025.
- Refrigerate specimens collected during this period until shipping is possible.
- We will test specimens received before 11:00 a.m. Tuesday, 12/23/2025 that same day.
- Results will be available by 5:00 p.m.
- We will test specimens received after 11:00 a.m. Tuesday, 12/23/2025 on Wednesday, 12/24/2025.
- We will notify submitters of positive results by 12:00 p.m. Preliminary results will be available by 12:00 p.m., if requested.
- Final results will be available to submitters on Monday, 12/29/2025 by 5:00 p.m.
- Emergency testing will be available Wednesday, 12/24/2025, Friday, 12/26/2025, and Thursday, 01/01/2026 only by prior arrangement with Rabies testing staff.
- Call 512-776-7595 before 4:00 p.m. of the previous day to discuss.
Emergency testing is not available on Thursday, 12/25/2025. - Specimens must be delivered by 8:00 a.m. the morning of testing.
- Preliminary results will be called out as soon as emergency testing is completed.
Tuberculosis (TB) Specimens
- Ensure critical AFB Smear and NAA for M. tuberculosis specimens are delivered by 8:30 a.m. Tuesday, 12/23/2025.
- Do not ship specimens for delivery between Wednesday, 12/24/2025 and Saturday, 12/27/2025.
- Refrigerate specimens, except for CSF, collected during this period until shipping is possible.
- Call 512-776-7342 before 9:00 a.m. on Tuesday 12/23/2025 for expedited test requests.
Candida auris Swab Specimens
- Do not collect or ship swabs for delivery before Monday, 01/05/2025.
Carbapenem Resistant Organism Swab Specimens
Do not collect or ship swabs from Tuesday, 12/23/2025 to Friday, 12/26/2025.
Neisseria gonorrhoeae Antimicrobial Susceptibility Specimens
Do not collect or ship swabs from Tuesday, 12/23/2025 to Monday, 12/29/2025 and Wednesday, 12/31/2025 to Friday, 1/2/2026.
- Swabs must be received within 24 hours of collection.
Bacteriology Water Samples
Water samples will not be accepted at DSHS Laboratory from Tuesday, 12/23/2025 to Friday, 12/26/2025 and Wednesday, 12/31/2025 to Friday, 1/2/2026.
All Other Microbiology Specimens or Samples
- Do not ship specimens for delivery between Wednesday, 12/24/2025 and Friday, 12/26/2025. Store specimens collected during this time at an appropriate temperature (e.g. -70°C or below for clinical virology samples).
- No testing will be performed from Wednesday, 12/24/2025 to Saturday, 12/27/2025.
Ordering Laboratory Supplies
TB/HIV/C. auris/Gonorrhea and Chlamydia Collection Kits/Courier Supply Order Requests
- Orders received before 9:00 a.m. on Tuesday, 12/23/2025 will be audited, issued, and shipped via FedEx Ground Monday afternoon, 12/29/2025.
- Orders received after 9:00 a.m. on Tuesday, 12/23/2025 through Sunday, 12/28/2025 will be audited, issued, and shipped on Friday, 01/02/2026 via FedEx Ground.
*** Per FedEx website, FedEx Ground will be closed Thursday, 12/25/2025 and Thursday, 1/1/2026. ***
Questions? Container Preparation: Environmental Chemistry Unit: Microbiology Unit: Microbiology General Inquiries: Specimen Submission: |
ContainerPrepGroup@dshs.texas.gov Lab.Microbiology@dshs.texas.gov 512-776-7599 |
Posted December 16, 2025
Important Update: Water Bacteriology Sample Collection and Form Changes, Effective January 5, 2026
Overview of Changes
The DSHS Austin and South Texas Laboratories are implementing crucial updates to the criteria for water bacteriology sample bottle acceptance and introducing a revised sample submission form. These changes are designed to enhance the accuracy and integrity of our testing process.
These updates will be effective beginning January 5, 2026.
Key Changes to Sample Collection and Submission
- Water Sample Bottles (New Acceptance Criteria):
Effective January 5, 2026, only IDEXX bottles approved and provided by the DSHS Austin and South Texas Laboratories will be accepted.
NOTE: Microtech Scientific bottles will not be accepted after the effective date.
- Expired bottles will no longer be accepted. Please verify the lot number (located on the bottom of the container) and expiration date in the tables below.
- Compliant sample containers are available for pickup from the DSHS Austin Laboratory and the South Texas Laboratory (STL).
- Required Submission Form (Revised G-19 Form):
- All water samples must be submitted using the revised G-19 Water Bacteriology Submission Form (Revision October 2025).
- Submitting a complete G-19 form is mandatory for sample processing.
Action Required
Please follow the mandatory changes to avoid sample rejection. We encourage all submitters to immediately transition to using the approved IDEXX bottles and the revised G-19 form for samples collected on or after January 5, 2026.
For additional details and a comprehensive review of all changes, please refer to the links provided below.
- DSHS Austin: Submitting Potable Water Samples to the Consumer Microbiology Laboratory | Texas DSHS
- DSHS South Texas: Public Drinking Water - Texas Commission on Environmental Quality - www.tceq.texas.gov
Table 1 Water Bacteriology Sample Collection Lots (AUSTIN)
| Lot Number: | Manufacturer: | Manufacturer's Expiration Date: |
| FY027V | IDEXX | 4/21/2027 |
| DY014V | IDEXX | 1/22/2027 |
| KW002V | IDEXX | 8/10/2026 |
Table 2 Water Bacteriology Sample Collection Lots (SOUTH TEXAS)
| Lot Number: | Manufacturer: | Manufacturer's Expiration Date: |
| AW249V | IDEXX | 12/07/2025 |
| CW013V | IDEXX | 02/07/2026 |
| CW015V | IDEXX | 02/12/2026 |
| KW002V | IDEXX | 08/10/2026 |
| DY014V | IDEXX | 01/22/2027 |
| EY011V | IDEXX | 03/07/2027 |
| GY015V | IDEXX | 05/01/2027 |
| JY015V | IDEXX | 06/17/2027 |
| B2001V | IDEXX | 02/11/2027 |
| AZ003V | IDEXX | 11/24/2027 |
| FZ169V | IDEXX | 05/04/2028 |
Posted December 03, 2025
DSHS Microbiology Laboratory Thanksgiving 2025
Closure Reminder
Except for the Rabies Lab, the DSHS Microbiology Laboratory will be closed, and no testing will occur Thursday, 11/27/2025 through Saturday, 11/29/2025. The DSHS Laboratory is closed on Sundays.
Testing services will resume as normal, Monday, 12/01/2025. See below for details on selected testing.
Rabies Specimens
- Do not ship specimens for delivery between Thursday, 11/27/2025 and Saturday, 11/29/2025.
- Refrigerate specimens collected during this period until shipping is possible.
- Rabies specimens received after 11:00 a.m. Wednesday, 11/26/2025 will be tested Friday, 11/28/2025.
- Submitters will be notified of positive results by 12:00 p.m. Friday.
- Ship specimens cold and overnight to best preserve the specimen. Do not freeze!
- Emergency testing will be available Friday, 11/28/2025 and Saturday, 11/29/2025 only by prior arrangement with Rabies testing staff.
- Call 512-776-7595 before 4:00 p.m. Wednesday, 11/26/2025 to discuss emergency testing.
- Specimens must be delivered by 8:00 a.m. the morning of testing. Preliminary results will be called out as soon as emergency testing is completed.
- Final results will be available Monday 12/01/2025.
- All other specimens will be tested Monday, 12/01/2025, and results will be available by 5:00 p.m.
Tuberculosis (TB) Specimens
- Ensure critical AFB Smear and NAA for M. tuberculosis specimens are delivered by 8:30 a.m. Wednesday, 11/26/2025.
- Do not ship specimens for delivery between Thursday, 11/27/2025 and Saturday, 11/29/2025.
- Refrigerate specimens, except for CSF, collected during this period until shipping is possible.
- Call 512-776-7342 before 9:00 a.m. on Wednesday, 11/26/2025 for expedited test requests.
Candida auris Swab Specimens
- Do not collect or ship swabs from Tuesday, 11/25/2025 to Saturday, 11/29/2025.
- Swabs must be received within 72 hours of collection.
Carbapenem Resistant Organism Swab Specimens
- Do not collect or ship swabs from Tuesday, 11/25/2025 to Saturday, 11/29/2025.
Neisseria gonorrhoeae Antimicrobial Susceptibility Specimens
- Do not collect or ship swabs from Tuesday, 11/25/2025 to Saturday, 11/29/2025.
- Swabs must be received within 24 hours of collection.
Bacteriology Water Samples
- Water samples will not be accepted at DSHS Laboratory from Wednesday, 11/26/2025 to Saturday, 11/29/2025.
All Other Microbiology Specimens or Samples
- Do not ship specimens for delivery between Thursday, 11/27/2025 and Sunday, 11/30/2025. Store specimens collected during this time at an appropriate temperature (e.g. -70°C or below for clinical virology samples).
- No testing will be performed from Thursday, 11/27/2025 to Sunday, 11/30/2025.
Ordering Laboratory Supplies
TB/HIV/Gonorrhea and Chlamydia Collection Kits/Courier Supply Order Requests
- Requests received before 9:00 a.m. on Wednesday, 11/26/2025 will be audited, issued, and shipped via FedEx Ground Wednesday afternoon, 11/26/2025.
- Requests received after 9:00 a.m. on Wednesday, 11/26/2025 through Sunday, 11/30/2025 will be audited, issued, and shipped on Monday, 12/01/2025 via FedEx Ground.
*** Per FedEx website, FedEx Ground will be closed Thursday, 11/27/2025 but will deliver packages
Friday, 11/28/2025.***
Questions?
Container Preparation: Environmental Chemistry Unit: Microbiology Unit: Microbiology General Inquiries: Specimen Submission: | ContainerPrepGroup@dshs.texas.gov Lab.Microbiology@dshs.texas.gov 512-776-7599 |
Posted: November 17, 2025
DSHS Austin Laboratory Veterans’ Day 2025 Closure
The DSHS Austin Microbiology Laboratory will be closed Tuesday, November 11.
Normal testing services will resume Wednesday, November 12.
- Do not ship or hand deliver microbiology specimens for Tuesday, November 11 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8:00 a.m. Tuesday, November 11.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Monday, November 10 from the Rabies Laboratory.
- Do not collect or ship gonorrhea antimicrobial susceptibility specimens on Monday 10, as these specimens must be tested within 24 hours of collection.
Questions about Shipping and the Holiday Closure?
Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
AR Lab Network: 512-776-7784 or texasarln@dshs.texas.gov
Specimen Intake Team: 512-776-7598 or 1-888-963-7111 ext. 7598 (toll free)
Posted: November 6, 2025
Resumption of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Real-Time RT-PCR
Testing at the DSHS Austin Laboratory
Effective Monday, 9/15/2025, the Texas DSHS Austin Laboratory resumed testing services to detect Middle East Respiratory Syndrome Coronavirus (MERS-CoV, or Novel Coronavirus 2012). Submitters must first notify their local epidemiologist or the state epidemiologist if MERS-CoV is suspected. The DSHS urges clinicians to promptly contact their local health department to coordinate shipping specimens to the appropriate public health laboratory.
For more information on notifiable conditions in Texas, please visit https://www.dshs.texas.gov/notifiable-conditions.
Refer to the MERS-CoV specimen submission one pager for additional guidance.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 2452.
Call Local: 512-776-2452
Email: viral.isolation@dshs.texas.gov
Posted: October 16, 2025
The Department of State Health Services (DSHS) Public Health Laboratory Division’s Antimicrobial Resistance (AR) Laboratory is temporarily halting free shipping through FedEx, except in limited circumstances. Any AR FedEx shipping labels created before September 1, 2025, are no longer valid and should be destroyed.
As of Monday, September 1, 2025, the CDC’s AR Lab Network (ARLN) FedEx account is no longer funded. The Texas AR Laboratory is in the process of transitioning to another FedEx account managed by the Association of Public Health Laboratories (APHL). Access to this account, and the ability to offer free shipping of AR samples, is expected to resume September 12, 2025. The Texas AR Laboratory is working to put temporary shipping methods in place until the new FedEx account becomes active.
DSHS is temporarily unable to provide free FedEx shipping labels for isolates or colonization swabs of the following organisms:
- Candida auris or unknown Candida spp.
- Carbapenem-Resistant Enterobacterales
- Carbapenem-Resistant Pseudomonas aeruginosa
- Carbapenem-Resistant Acinetobacter baumannii
- Antimicrobial Resistant Neisseria gonorrhoeae (AR GC)
There is no interruption to testing for these organisms. DSHS can receive specimens delivered via courier or other shipping methods. However, APHL and CDC cannot reimburse for those shipping costs. During this transitionary period, we are accepting batched specimens to conserve costs for submitters that self-pay for shipment or are using alternative funding sources.
If you have a significant investigation or emergency circumstance, please contact TexasARLN@dshs.texas.gov to determine if alternative funding can be used for shipping your samples to the Texas AR Laboratory.
Posted: September 5, 2025
DSHS Austin Microbiology Laboratory Closed Labor Day 2025
The DSHS Austin Microbiology Laboratory will be closed Monday, September 1, 2025 in observance of Labor Day. Normal testing services will resume Tuesday, September 2, 2025.
- Do not ship or hand deliver microbiology specimens for Monday, September 1 delivery.
- Emergency rabies testing will be available only for specimens dropped off before 8:00 a.m. Monday, September 1.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, August 29 from the Rabies Laboratory.
Do not collect or ship gonorrhea antimicrobial susceptibility specimens on Sunday, August 30 as these specimens must be tested within 24 hours of collection.
Questions? Contact the DSHS Austin Laboratory:
- AR Lab Network: 512-776-7784 or texasarln@dshs.texas.gov
- Measles Specimens/Testing Questions: 512-776-7594 or 512-776-7760,
or viral.isolation@dshs.texas.gov - Measles Surveillance: EAIDUMeasles2025@dshs.texas.gov and Measles (Rubeola) | Texas DSHS
- Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
- Specimen Intake Team: 512-776-7598 or 1-888-963-7111
ext. 7598 (toll free) - TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
Posted: August 29, 2025
DSHS Laboratory TB Specimen Cold Box Program
DSHS Austin Laboratory – Microbiological Sciences Unit Notice
Local and regional health departments (HDs) that receive state funding from the DSHS Tuberculosis and Hansen’s Disease Unit (TB Unit) or similar support from DSHS Public Health Regions for their TB programs are required to submit patient specimens to the DSHS Laboratory for testing.
Providers must submit sputum specimens for TB testing to the DSHS Laboratory as soon as possible after collection. Shipping the sputum specimens cold and overnight helps protect their integrity. While shipping these specimens at ambient temperatures is acceptable, submitting them cold and overnight minimizes the growth of background organisms and optimizes result turn-around-times.
The DSHS Cold Box Program offers insulated cooler boxes, cardboard outer mailers, and gel ice packs for submitting sputum specimens cold for TB testing. Guidance on how to sign up for and order from the DSHS Cold Box Program is available on the DSHS Specimen Submission Job Aids webpage.
Caution: Specimens submitted ambient via USPS may take up to a week to arrive at the Laboratory, which compromises specimen quality and delays test results.
Questions about the TB Specimen Cold Box Program?
TB Clinical Group Manager: 512-776-7586
Mycobacteriology Branch Manager: 512-776-7342
Submitting TB Specimens: Submitting Mycobacterium Specimens to DSHS Laboratory | Texas DSHS
DSHS Pharmacy Inventory and Ordering System Registration: Home - Texas DSHS Pharmacy Providers Enrollment Site
Overnight/Priority Shipping Address
Texas Department of State Health Services
Public Health Laboratory Division
1100 W. 49th Street
Austin, TX 78756-3199
Posted: August 26, 2025
****Effective Immediately****
Measles IgM Testing Information
Due to delays in the manufacture of Measles IgM EIA kits, the DSHS Laboratory is temporarily unable to perform Measles IgM testing. During this time, all specimens received at DSHS Laboratory requesting this test will be forwarded to Quest Diagnostics laboratory for processing.
Measles IgM test results will continue to be reported from DSHS Laboratory but will include an additional attached report displaying the Quest results. Please continue to submit specimens for Measles IgM testing to DSHS Laboratory following specimen submission requirements.
The Laboratory will release a follow-up notification when the issue is resolved.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 7760
Call Local: 512-776-7760
Email: Lab.Microbiology@dshs.texas.gov
Posted: August 26, 2025
****Effective Immediately****
DSHS Laboratory Resumes Measles IgM Testing
The DSHS Serology Laboratory is pleased to announce the resumption of Measles IgM serologic testing. Healthcare providers are encouraged to continue submitting specimens for Measles IgM testing in accordance with the DSHS specimen submission guidelines.
Measles Specimen Submission Guidance is available on the DSHS Specimen Submission Job Aids webpage
We thank you for your commitment to keeping Texans healthy.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 7760
Call Local: 512-776-7760; 512-776-7657; 512-776-2505
Email: Lab.Microbiology@dshs.texas.gov
Posted: July 08, 2025
****Effective Immediately****
Rickettsia Detection PCR Testing Services Discontinued
Effective June 1, 2025, the Texas DSHS Austin Laboratory is no longer providing Rickettsia detection by real-time PCR.
If specimens for the Rickettsia PCR assay are received at the DSHS Laboratory on or after June 1, 2025, Laboratory staff will forward them to the CDC for testing at no cost to the submitter. Rickettsia PCR testing at the CDC has a projected turnaround time of six weeks.
The DSHS Laboratory will provide test results to submitters for CDC Rickettsia PCR testing as an attachment to the DSHS patient report.
Rickettsia PCR Testing Questions or Comments?
Call Toll-Free: 1-888-963-7111 ext. 2452.
Call Local: 512-776-2452
Email: viral.isolation@dshs.texas.gov
Posted: July 08, 2025
DSHS Austin Laboratory Independence Day Closure
The DSHS Austin Microbiology Laboratory will be closed Friday, July 4.
- Do not ship microbiology specimens for Friday, July 4 delivery.
- Emergency rabies testing is available only for specimens dropped off by 8 a.m. Friday, July 4.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Thursday, July 3 from the Rabies Laboratory.
- TB Elimination submitters should not collect or ship specimens on Thursday, July 3. Due to the extreme heat, ice packs will not remain frozen over the holiday. Specimen quality may be affected, causing specimens to be rejected.
- Do not collect or ship gonorrhea antimicrobial susceptibility specimens Thursday, July 3. These specimens must be tested within 24 hours of collection.
The Microbiology Laboratory will resume normal business hours Monday, July 7.

SUMMER HEAT REMINDER: Please ship refrigerated specimens with multiple ice packs and frozen specimens with enough dry ice to keep them cold/frozen for up to 48 hours. Improve insulation and cushioning of specimen by packing empty space in shipping container with absorbent material.
Questions about Shipping and the Holiday Closure?
Rabies Team: (512) 776-7595 or rabies.team@dshs.texas.gov
TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
Specimen Intake Team: (512) 776-7598 or 1-888-963-7111
ext. 7598 (toll free)
Posted: July 01, 2025
DSHS Public Health Laboratory– Measles and Rabies Testing Notice
Due to ongoing critical generator updates and repairs, this Saturday, May 31, 2025, the Texas Department of State Health Services Public Health Laboratory in Austin, Texas will not be testing measles or rabies samples. Samples received on Saturday, May 31, will get processed on Monday, June 2. Please follow appropriate shipping guidelines.
Questions? Contact the DSHS Austin Laboratory:
- Specimen Submissions: LabInfo@dshs.texas.gov
- Measles Specimens/Testing Questions: 512-776-7594 or 512-776-7760, or viral.isolation@dshs.texas.gov
- Rabies Specimens/Testing Questions: 512-776-7595
- Specimen Submission Guidance available on the DSHS Specimen Submission Job Aids webpage.
Posted: May 27, 2025
DSHS Austin Microbiology Laboratory Closed Memorial Day 2025
The DSHS Austin Microbiology Laboratory will be closed Monday, May 26, 2025, in observance of Memorial Day. Normal testing services will resume Tuesday, May 27.
- Do not ship or hand deliver microbiology specimens for Monday, May 26 delivery.
- Emergency rabies testing will be available only for specimens dropped off before 8:00 a.m. Monday, May 26.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, May 23 from the Rabies Laboratory.
- Do not collect or ship gonorrhea antimicrobial susceptibility specimens on Sunday, May 25 as these specimens must be tested within 24 hours of collection.
Questions? Contact the DSHS Austin Laboratory:
- AR Lab Network: 512-776-7784 or texasarln@dshs.texas.gov
- Measles Specimens/Testing Questions: 512-776-7594 or 512-776-7760, or viral.isolation@dshs.texas.gov
- Measles Surveillance: EAIDUMeasles2025@dshs.texas.gov and Measles (Rubeola) | Texas DSHS
- Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
- Specimen Intake Team: 512-776-7598 or 1-888-963-7111
ext. 7598 (toll free) - TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
Posted: May 23, 2025
DSHS Public Health Laboratory– Measles Testing Notice
Due to ongoing critical generator updates and repairs, this Saturday, May 17, 2025, the Texas Department of State Health Services Public Health Laboratory in Austin, Texas will not be testing measles samples. Samples received on Saturday, May 17th, will be held until Monday, May 19th. Please follow appropriate shipping guidelines.
Questions? Contact the DSHS Austin Laboratory:
- Specimen Submissions: LabInfo@dshs.texas.gov
- Measles Specimens/Testing Questions: 512-776-7594 or 512-776-7760, or viral.isolation@dshs.texas.gov
- Specimen Submission Guidance available on the DSHS Specimen Submission Job Aids webpage.
Posted: May 15, 2025
Resumption of Regular Hepatitis Serology Testing Processes
The DSHS Austin Laboratory has resumed regular testing for Hepatitis B (HBV) Surface Antibody and Hepatitis A (HAV) IgM using Bio-Rad's MONOLISA Anti-HBs EIA and MONOLISA Anti-HAV IgM EIA kits.
Please continue to submit specimens for HBV and HAV serology testing to the DSHS Austin Laboratory following specimen submission requirements.
Thank you for your patience and commitment to keeping Texans healthy.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 2505
Call Local: 512-776-2505
Email: serological.analysis@dshs.texas.gov
Posted: May 01, 2025
DSHS Austin Microbiology Laboratory Closed Due to Construction Work, Saturday and Sunday April 26 and 27, 2025
The DSHS Microbiology Laboratory in Austin will be closed Saturday and Sunday April 26 and 27 due to the ongoing construction work. No testing will occur during the weekend closure, no exceptions.
Emergency rabies testing and measles testing will be unavailable during the closure. The Laboratory will reopen at 8:00 a.m. on Monday, April 28.
Please Note:
- Do not ship rabies or measles specimens to the Laboratory for delivery on Saturday, April 26.
- Do not hand-deliver rabies or measles specimens to the Laboratory on Saturday, April 26.
- Specimens received outside of their acceptability window will be unsatisfactory for testing.
The Laboratory will work at maximum capacity to resolve any specimen backlog.
Questions? Contact the DSHS Austin Laboratory:
- Specimen Submissions: LabInfo@dshs.texas.gov
- Measles Specimens/Testing Questions: 512-776-7594 or 512-776-7760, or viral.isolation@dshs.texas.gov
- Rabies Specimens/Testing Questions: 512-776-7595
- Specimen Submission Guidance available on the DSHS Specimen Submission Job Aids webpage.
Posted: April 25, 2025
****Effective Immediately****
Temporary Change to Hantavirus, IgG, and IgM Enzyme Immunoassay Testing Process
Due to unexpected issues, the DSHS Laboratory is temporarily unable to perform the Hantavirus IgG and IgM serology test. During this time, the Laboratory will forward all specimen submissions requesting this diagnostic test to a reference laboratory for testing.
The DSHS Laboratory will continue to provide Hantavirus IgG and IgM test results to submitters. Patient reports will include an attachment with the external testing laboratory’s results. Turnaround times for Hantavirus IgG and IgM results may be longer than usual while the specimens are being tested at the external laboratory.
Please continue to submit specimens for Hantavirus IgG and IgM Enzyme Immunoassay Serology Testing to the DSHS Laboratory following specimen submission requirements.
The DSHS Laboratory will release a follow-up notification when this issue is resolved.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 2505.
Call Local: 512-776-2505
Email: Serological.Analysis@dshs.texas.gov
Posted: April 11, 2025
Delays in Serology Screen Testing Turnaround Times Due to Supply Chain Issues
Due to unexpected delays in the manufacture of Bio-Rad's MONOLISA Anti-HBs EIA and MONOLISA Anti-HAV IgM EIA kits, effective Tuesday, March 25, 2025, the DSHS Laboratory will begin forwarding specimens submitted for both Hepatitis B Surface Antibody and Hepatitis A IgM testing to a reference laboratory until the supply issue is resolved. All other hepatitis testing is unaffected by this manufacturing issue. The DSHS Laboratory will continue conducting the unaffected tests and reporting their results as usual.
The DSHS Laboratory will continue providing results for the Hepatitis B Surface Antibody and Hepatitis A IgM tests to submitters. Patient reports for these tests will include an attachment with the external testing laboratory’s results. Turnaround times for Hepatitis B Surface Antibody and Hepatitis A IgM results may be longer than usual while the specimens are being tested at the external laboratory. We will provide an update when the supply issue is resolved.
Questions?
Contact the Serology Laboratory
Call: 512-776-7657 or 512-776-2505 or 1-888-963-7111 ext. 2505 (toll-free)
Email: serological.analysis@dshs.texas.gov
Posted March 31, 2025
Request for Faster Subtyping of In-Patient Influenza A Positive Specimens
In a recent CDC Health Alert released January 16, 2025, the CDC recommended all influenza A positive specimens from hospitalized patients be subtyped as soon as possible, ideally within 24 hours of admission.
Recommendations for Clinicians
Subtyping should be carried out in the hospital laboratory, if available, or at a commercial laboratory. If subtyping is not available through a hospital or commercial lab, the DSHS urges clinicians to promptly contact their local health department to coordinate shipping specimens to the appropriate public health laboratory for subtyping.
Recommendations for Clinical Laboratories
Clinical laboratories that subtype should submit influenza A positive specimens that are negative for seasonal influenza A virus subtypes A(H1) and A(H3) to their local public health laboratory within 24 hours of subtyping results.
Submission Recommendations
If you are going to submit influenza specimens to the DSHS Austin Laboratory, please complete a
G-2V specimen submission form. Indicate in Section 4 of the G-2V form whether:
- the patient was exposed to infected wild or domestic animals with confirmed or suspected influenza A (H5N1),
- the patient experienced a high-risk exposure to H5N1, or
- subtyping results were negative for seasonal influenza A virus subtypes.
Please also refer to the attached Flu job aid for additional influenza specimen submission instructions and FAQs on submitting specimens.
Subtyping specimens as soon as possible will help to quickly identify human infections with avian influenza A(H5) viruses and routinely characterize seasonal influenza viruses.
Influenza Testing, Specimen Submission, or Sequencing Questions?
- Influenza Testing: 512-776-7594, 512-776-2452 or viral.isolation@dshs.texas.gov
- Influenza and Influenza-Like Illness Surveillance: flutexas@dshs.texas.gov
- Submitter ID Numbers or Submission Forms: 512-776-7578 or LabInfo@dshs.texas.gov
- Overnight Shipping Address: Walter Douglass, Texas Dept. of State Health Services, Public Health Laboratory Division, 1100 W. 49th Street, Austin, TX 78756-3199
Posted February 13, 2025
DSHS Austin Laboratory Open Regular Business Hours - Wednesday, January 22, 2025
The DSHS Austin Laboratory will return to normal operations Wednesday, January 22, 2025.
We understand that some areas may still be negatively affected by cold and icy conditions so please check with your courier for local service alerts. Courier services may experience delays due to prolonged cold or hazardous conditions.
Received specimens will be tested as soon as possible, and backlogs will be resolved during business hours.
- Please Note: Specimens received outside of their acceptability window will be unsatisfactory for testing.
Questions? Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted January 21, 2025
DSHS Austin Laboratory Closure Due to Inclement Weather January 21, 2025
Inclement weather in Austin and other parts of Texas on Tuesday, January 21, 2025, may cause testing delays at the Laboratory.
- The DSHS Austin Microbiology Unit and Environmental Sciences Laboratory will be closed on
Tuesday, January 21, 2025, due to inclement weather and hazardous road conditions. - The Newborn Screening Laboratory will perform limited testing during that time.
- Continue to ship all specimens per regular guidelines.
- Due to the potential for shipping delays, please transfer serum for glucose and lipid testing from SST tubes into new tubes and freeze them. Then ship them only after weather conditions improve.
- Courier services may experience delays due to the weather.
- Please check with your courier for local service alerts.
- All specimens will be processed as soon as possible after resumption of normal operations.
- Note that specimens received outside of their acceptability window will be unsatisfactory for testing.
- The Laboratory will work at maximum capacity to resolve any specimen backlog.
The DSHS Austin Laboratory appreciates the dedication and commitment of healthcare providers to keep Texans healthy.
Contact the DSHS Austin Laboratory:
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted January 20, 2025
DSHS Austin Microbiology Laboratory Closed on Martin Luther King, Jr. Day
The DSHS Austin Microbiology Laboratory will be closed Monday, January 20 in observance of Martin Luther King, Jr. Day.
- Do not ship microbiology specimens for Monday, January 20 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8 a.m. Monday, January 20.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, January 17 from the Rabies Laboratory.
- TB Elimination submitters should not collect or ship specimens on Friday, January 17.
- Do not collect or ship gonorrhea antimicrobial susceptibility (GC AST) specimens Sunday, January 19 as these specimens must be tested within 24 hours of collection.
- As is standard practice, please do not collect or ship GC AST specimens on Friday for Saturday delivery.
The Microbiology Laboratory will resume normal business hours Tuesday, January 21.
Questions about Shipping and the Holiday Closure?
Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
TB Team: 512-776-7342 or mycobacteriology@dshs.texas.gov
Specimen Intake Team: 512-776-7598 or 1-888-963-7111 ext. 7598 (toll free)
Posted January 16, 2025
Request for Antimicrobial Resistant Sample Submissions
Dear Laboratory managers, microbiology supervisors, and infection preventionists,
The prevalence of antimicrobial resistant bacteria and fungi is an urgent global public health challenge that can threaten the health and livelihoods of Texans. The CDC’s Antimicrobial Resistance Threats in the United States, 2021-2022 report analyzed new data showing that seven antimicrobial resistant pathogens increased the burden of hospital onset infections by a combined 20% during the COVID-19 pandemic compared to the pre-pandemic period. To ensure healthcare facilities have processes in place to prevent other patients from contracting these pathogens, Texas epidemiologists use the containment guidance in the Texas Antimicrobial Resistance Laboratory Network Response Plan.
The Texas Antimicrobial Resistance (AR) Laboratory, as part of the CDC’s AR Laboratory Network, works with local and regional healthcare facilities and laboratories across the state to rapidly detect antimicrobial resistant microbes and prevent their spread. You can help by submitting swabs and/or isolates of the following microbes to the Texas AR Laboratory:
- *Candida auris or unknown Candida spp.
- Carbapenem-Resistant Enterobacterales
- Carbapenem-Resistant Pseudomonas aeruginosa
- Carbapenem-Resistant Acinetobacter baumannii
- Antimicrobial Resistant Neisseria gonorrhoeae (AR GC)
*It is mandatory to submit isolates for positive cases of C. auris per Texas Administrative Code Rule §97.3.
Texas AR Laboratory tests submissions for organism identification, antimicrobial susceptibility, carbapenemase production, and mechanism of resistance. Additional details about AR Lab Network testing can be found in the Texas Antimicrobial Resistance Laboratory Network Response Plan.
Contact Information
Texas AR Laboratory - AR Testing, Specimen Submissions
Multidrug-resistant Organisms (MDRO) Group - AR Organisms, Data
Healthcare Associated Infections (HAI) Team – Screenings, ICARs
Shipping Information More details about shipping specimens for antimicrobial resistance testing may be found online at the links below.
- Texas Antimicrobial Resistance Laboratory
- Guidelines for Specimen Collection and Submission
- Guidelines for Specimen Shipping and Mailing
- Lab Specimen Receiving, Lab Hours, and Holidays
Thank you for your partnership with us in this crucial public health effort for all Texans.
Sincerely,
The Texas Antimicrobial Resistance Laboratory and
the Texas Healthcare Associated Infections/ Antimicrobial Resistance Investigations Group
Posted December 30, 2024
Request for Norovirus Specimen Submitters to Select “Outbreak” or “Surveillance” on G-2B Submission Forms

The DSHS Austin Laboratory requests that submitters of norovirus positive (NoV+) clinical specimens mark on the G-2B submission form whether the specimen is linked to an outbreak investigation or submitted for surveillance purposes. Doing so helps the Laboratory and epidemiologists better track and follow up on all submitted NoV specimens.
What is the Difference Between Outbreak and Surveillance Specimens?
During outbreak investigations, your local or regional health department may ask you to forward clinical specimens to the DSHS Lab for molecular analysis. Select “Outbreak Association” in Section 2 of the G-2B submission form for these specimens. We highly encourage the submission of at least two NoV specimens from two different patients for confirmed outbreaks. But do not hesitate to submit single NoV+ specimens suspected of being part of an outbreak.
Surveillance specimens are specimens your local or regional health department did not prompt or request you to submit to the Lab. They are not linked to an outbreak investigation. Please select “Surveillance” in Section 2 of the G-2B submission form for these specimens.
Thank you so much for the positive response to our prior request for norovirus specimens. Keep up the great work!
Additional Guidance
Norovirus specimen submission job aids are available.
Shipping Address for Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Public Health Laboratory Division,
Department of State Health Services
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7569
Norovirus Questions?
For laboratory questions about NoV testing or specimen suitability, please contact the Molecular Biology Team Lead at (512) 776-6510.
For questions related to the DSHS NoV surveillance project (CaliciNet), please email FoodborneTexas@dshs.texas.gov.
For questions about G-2B submission forms, contact the Lab Reporting Team at 512-776-7578 or LabInfo@dshs.texas.gov.
Posted December 27, 2024
DSHS Austin Laboratory Christmas and New Year’s Holiday Closure Reminder
With the exception of Newborn Screening and Rabies testing, the DSHS Austin Laboratory will be closed, and testing will not occur Tuesday, 12/24/2024, Wednesday, 12/25/2024, and Thursday, 12/26/2024. Testing services will resume as normal, Friday, 12/27/2024.
- The Laboratory will be closed on New Year’s Day, Wednesday 01/01/2025.
All submitters are responsible for the timely and appropriate shipping of specimens to the Laboratory.
See below for more details on selected testing.
Newborn Screening (NBS)
Christmas Closure is Tuesday, 12/24/2024 and Wednesday, 12/25/2024. Testing will resume Thursday, 12/26/2024.
New Year’s Closure is Wednesday, 01/01/2025. Testing will resume Thursday, 01/02/2025.
- We will test NBS specimens received and accessioned on Monday, 12/23/2024 and Tuesday, 12/31/2024.
- We will notify healthcare providers of critical-level preliminary presumptive positive results on Tuesday, 12/24/2024 (Christmas Eve).
Submission Reminders
- DO NOT DELAY the collection or shipment of newborn screening specimens.
- Ship dried specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
- DSHS recommends shipping specimens using an overnight or trackable service such as USPS Priority Mail, FedEx, and UPS.
Clinical Chemistry
Christmas Closure is Tuesday, 12/24/2024 through Thursday, 12/26/24. Testing will resume Friday, 12/27/2024.
New Year’s Closure is Wednesday, 01/01/2025. Testing will resume Thursday, 01/02/2025.
- Total hemoglobin, lead, glucose, and lipid/cholesterol specimens must be received by 12:00 p.m. on Monday, 12/23/2024 to complete testing before the Holiday closure.
- DO NOT ship cholesterol, lipid profile, or glucose specimens on Friday, 12/27/2024.
Submission Reminders
- Specimens submitted for lead and total hemoglobin testing must be received within 14 days of collection.
- Specimens submitted for glucose, lipid panel, and cholesterol testing must be received according to the specimen submission guidelines available at
Laboratory Testing Services Manual - Guidelines for Specimen Shipping and Mailing | Texas DSHS.
Environmental Science
- If you require any priority analysis during the holiday period, please reach out to envsciadmin@dshs.texas.gov.
Microbiology
Christmas Closure is Tuesday 12/24/2024 through Thursday 12/26/2024. Testing will resume Friday 12/27/2024.
New Year’s Closure is Wednesday 01/01/2025. Testing will resume Thursday 01/02/2025.
Rabies Specimens
- We will test specimens received before 11:00 a.m. Monday, 12/23/2024 the same day.
- Results will be available by 5:00 p.m.
- We will test specimens received after 11:00 a.m. Monday, 12/23/2024 on Tuesday, 12/24/2024.
- We will notify submitters of positive results by 12:00 p.m. Preliminary results will be available by 12:00 p.m., if requested.
- Final results will be available to submitters on Friday, 12/27/2024 by 5:00 p.m.
- Emergency testing—only by prior arrangement with Rabies testing staff—will be available: Tuesday, 12/24/2024, Thursday, 12/26/24; and Wednesday, 01/01/2025.
- Call 512-776-7595 before 4:00 p.m. of the previous day to discuss.
- Emergency testing is not available on Wednesday, 12/25/2024.
- Specimen receipt by 8:00 a.m. must be guaranteed to ensure same day testing and preliminary results.
- DO NOT ship specimens for delivery Tuesday, 12/24/2024 through Thursday, 12/26/2024 or Wednesday, 01/01/2025. Store specimens at 2ºC–8ºC until shipping is possible.
- All other specimens will be tested on Friday, 12/27/2024.
Tuberculosis (TB) Specimens
- The TB Lab must receive critical specimens for AFB Smear and NAA testing by 8:30 a.m., Monday, 12/23/2024 for expedited AFB Smear and NAA test results before the Christmas holiday.
- DO NOT ship specimens for delivery on Tuesday, 12/24/2024 through Thursday, 12/26/2024.
- Refrigerate specimens collected during this period until shipping is possible.
- Call 512-776-7342 before 9:00 a.m., Monday, 12/23/2024 for expedited test requests.
Candida auris Swab Specimens
- Do not ship swabs for delivery on Monday, 12/23/2024 through Wednesday, 1/1/2025.
- Please remember that swabs must be received within 72 hours of collection.
Carbapenem Resistant Organisms Swab Specimens
- Do not collect or ship swabs from Monday, 12/23/2024 through Friday 12/27/2024.
- Do not ship swabs for delivery on Wednesday, 1/1/2025.
Neisseria gonorrhoeae Antimicrobial Susceptibility Testing Specimens
- Do not collect or ship swabs from Monday, 12/23/2024 through Thursday, 12/26/2024.
Bacteriology Water Samples
- Do not ship for delivery or drop off samples from Monday, 12/23/2024 to Friday, 12/27/2024.
- We will accept water samples on Monday, 12/30/2024.
- Do not ship for delivery or drop off samples on Tuesday, 12/31/2024 or Wednesday, 01/01/2025.
- Our regular specimen acceptance schedule will resume on Thursday, 01/02/2025.
All Other Microbiology Specimens or Samples
- Do not ship specimens for delivery between Tuesday, 12/24/2024 and Thursday, 12/26/2024.
- Store specimens collected during this time at an appropriate temperature (e.g., -70°C) or below, for clinical virology samples.
- No tests will be performed from Tuesday, 12/24/2024 to Thursday, 12/26/2024.
Ordering Laboratory Supplies
Newborn Screening Requests
- Priority overnight orders received by 12:00 p.m. Monday, 12/23/2024 will be processed and shipped via FedEx Priority Overnight.
- Standard orders received by 12:00 p.m. Monday, 12/23/2024 will be processed and shipped via FedEx Ground.
- Orders received after 12:00 p.m. on Monday, 12/23/2024 through Thursday, 12/26/2024 will be processed on Friday, 12/27/2024.
Texas Health Steps/PKU/TB/HIV/C. auris/CRO/GC AST Courier Supply Requests
- Orders received on Monday, 12/23/2024 will be audited, issued, and shipped via FedEx Ground on Friday, 12/27/2024.
- Orders received on Tuesday 12/31/2024 will be audited, issued, and shipped on Thursday 01/02/2024 via FedEx Ground.
***Per FedEx website, FedEx Ground will be closed Wednesday, 12/25/2024. FedEx Ground will also be closed Wednesday, 01/01/2025.***
Questions?
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted December 11, 2024
DSHS Austin Laboratory Thanksgiving Holiday Closure Reminder
With the exception of Newborn Screening and Rabies testing, the DSHS Austin Laboratory will be closed, and no testing will occur Thursday, 11/28/2024 through Saturday, 11/30/2024. The DSHS Laboratory is completely closed on Sundays. Testing services will resume as normal, Monday, 12/02/2024.
See below for more details on selected testing.
Newborn Screening (NBS)
- The Newborn Screening Laboratory will be closed Thanksgiving Day, Thursday 11/28/2024 and will resume testing on Friday, 11/29/2024.
- DO NOT DELAY collection or shipment of newborn screening specimens.
- Ship dried specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
- DSHS recommends shipping specimens using an overnight or trackable service like USPS Priority Mail, FedEx, and UPS.
Clinical Chemistry
- Total hemoglobin, lead, glucose, and lipid/cholesterol specimens must be received by Wednesday, 11/27/2024 to complete testing before the holiday closure.
- DO NOT ship cholesterol, lipid profile, or glucose specimens on Fridays or on the day before a state-observed holiday.
Microbiology
Rabies Specimens
- Rabies specimens received after 11:00 a.m. Wednesday, 11/27/2024 will be tested Friday, 11/29/2024. Submitters will be notified of positive results by 12:00 p.m. Friday.
- All other results will be available Monday 12/02/2024.
- Emergency testing will be available Friday, 11/29/2024 and Saturday, 11/30/2024 only by prior arrangement with Rabies testing staff. Call 512-776-7595 before 4:00 p.m. Wednesday, 11/27/2024 to discuss.
- Specimen receipt must be guaranteed by 8:00 a.m. the morning of testing. Preliminary results will be called out as soon as emergency testing is completed. Final results will be available Monday 12/02/2024.
- All other specimens will be tested on Monday, 12/02/2024, and results will be available by 5:00 p.m.
Tuberculosis (TB) Specimens
- Do not ship specimens for delivery between Thursday, 11/28/2024 and Saturday, 11/30/2024.
- Refrigerate specimens collected during this period until shipping is possible.
- Ensure critical AFB Smear and NAA for M. tuberculosis specimens are delivered by 8:30 a.m. Wednesday, 11/27/2024.
- Call 512-776-7342 before 9:00 a.m. on Wednesday, 11/27/2024 for expedited test requests.
Candida auris Swab Specimens
- Do not collect or ship swabs for delivery from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
- Please remember that swabs must be received within 72 hours of collection.
- Carbapenem Resistant Organisms Swab Specimens
- Do not collect or ship swabs from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
- Neisseria gonorrhoeae Antimicrobial Susceptibility Testing Specimens
- Do not collect or ship swabs from Tuesday, 11/26/2024 to Saturday, 11/30/2024.
Bacteriology Water Samples
- Water samples will not be accepted at DSHS Laboratory from Wednesday, 11/27/2024 to Saturday, 11/30/2024.
All Other Microbiology Specimens or Samples
- Do not ship specimens for delivery between Thursday, 11/28/2024 and Sunday, 12/01/2024. Store specimens collected during this time at an appropriate temperature (e.g. -70°C or below for clinical virology samples).
- No testing will be performed from Thursday, 11/28/2024 to Sunday, 12/01/2024.
Ordering Laboratory Supplies
Newborn Screening Medicaid/Paid Card Order Requests
- Priority overnight shipping requests received on Wednesday, 11/27/2024 will be processed and shipped via FedEx Priority Overnight.
- All requests received before noon Wednesday, 11/27/2024 will be processed and shipped via FedEx Ground.
- Requests received after noon Wednesday, 11/27/2024 through Saturday, 11/30/2024 will be sent Monday, 12/02/2024.
Texas Health Steps/PKU/TB/HIV/Courier Supply Order Requests
- Requests received before 9:00 a.m. on Wednesday, 11/27/2024 will be audited, issued, and shipped via FedEx Ground Wednesday afternoon, 11/27/2024.
- Requests received after 9:00 a.m. on Wednesday, 11/27/2024 through Sunday, 12/01/2024 will be audited, issued, and shipped on Monday, 12/02/2024 via FedEx Ground.
*** Per FedEx website, FedEx Ground will be closed Thursday, 11/28/2024 but will deliver packages Friday, 11/29/2024.***
Questions?
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Clinical Chemistry General Inquiries: 512-776-6236
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Environmental Chemistry Unit: EnvSciAdmin@dshs.texas.gov
Microbiology Unit: Lab.Microbiology@dshs.texas.gov
Microbiology General Inquiries: 512-776-7599
Newborn Screening General Inquiries: 512-776-7333
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Posted November 14, 2024
DSHS Austin Laboratory Veterans’ Day 2024 Closure
The DSHS Austin Microbiology Laboratory will be closed Monday, November 11. Normal testing services will resume Tuesday,
November 12.
- Do not ship or hand deliver microbiology specimens for Monday, November 11 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8:00 a.m. Monday, November 11.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Friday, November 8 from the Rabies Laboratory.
- Do not collect or ship gonorrhea antimicrobial susceptibility specimens on Sunday, November 10 as these specimens must be tested within 24 hours of collection.
Questions about Shipping and the Holiday Closure?
Rabies Team:
512-776-7595 or rabies.team@dshs.texas.gov
TB Team:
512-776-7342 or mycobacteriology@dshs.texas.gov
AR Lab Network:
512-776-7784 or texasarln@dshs.texas.gov
Specimen Intake Team:
512-776-7598 or 1-888-963-7111
ext. 7598 (toll free)
Posted November 07, 2024,
Norovirus Specimens in Cary Blair Transport Medium Now Accepted for Molecular Analysis at DSHS Austin Laboratory
Effective 11/1/2024, the Texas Department of State Health Services Austin Laboratory is accepting frozen raw stool and stool specimens in Cary-Blair transport medium for norovirus (NoV) molecular analysis. The Laboratory continues to accept refrigerated raw stool specimens for NoV analysis.
Norovirus outbreaks tend to be underreported to public health officials, so the true incidence of this foodborne illness is likely higher. Testing patients for unknown gastrointestinal illnesses is essential for clinical decision-making and for community outbreak detection and investigations.
During outbreak investigations, your local or regional health departments may request you to forward clinical specimens to the DSHS Laboratory for molecular analysis. It is essential that you submit requested specimens to help DSHS epidemiologists link individual cases during outbreak investigations.
Norovirus Analysis is FREE when requested by an epidemiologist. There is no charge to the submitter for testing clinical specimens that were requested for NoV outbreak investigation purposes.
Refer to the printable Norovirus one-page flyers for more guidance on collecting and shipping NoV specimens to the Laboratory for molecular analysis.
Shipping Address for Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Public Health Laboratory Division,
Department of State Health Services,
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7569
Norovirus Questions?
For questions about NoV testing or specimen suitability, please contact the Molecular Biology Team Lead at 512-776-6510.
For questions related to DSHS’ NoV surveillance project (CaliciNet), please email FoodborneTexas@dshs.texas.gov.
For more information on specimen packaging and shipping, check out the shipping and submission guidance webpage.
Posted October 30, 2024
DSHS Austin Laboratory Phone and Fax Services Technical Issues
The DSHS Austin Laboratory is experiencing intermittent technical issues with outbound and inbound phone service and outbound fax systems. We are currently unable to receive and make phone calls and send faxes to various areas of Texas. The issue is being investigated and evaluated for resolution.
In the interim, the Laboratory is using alternative means to contact our submitters. Alternatively, please use email to initiate communication directly with your Laboratory contact.
| DSHS Austin Laboratory Contact | Phone Number | |
|---|---|---|
| AR Laboratory Network | TexasARLN@dshs.texas.gov | |
| AR Lab Network Liaison | 512-776-2321 | kimberly.abella@dshs.texas.gov |
| BioThreat Team | 512-776-3781 | dshslrn@dshs.texas.gov |
| Chemical Threat Team | 512-776-7270 | dshslrnc@dshs.texas.gov |
| Clinical Chemistry Educator | 512-776-6236 | jadyn.pena@dshs.texas.gov |
| Clinical Chemistry Specimen Logistics | 512-776-6237 or 512-776-2628 | clinicalchemistry@dshs.texas.gov |
| Container Supply/Kit Ordering | 512-776-7661 | ContainerPrepGroup@dshs.texas.gov |
| Environmental Chemistry Unit | 512-776-7587 | EnvSciAdmin@dshs.texas.gov |
| Laboratory Reporting Group | 512-776-7578 | labinfo@dshs.texas.gov |
| Microbiology Laboratory Educator | 512-776-3877 | niamh.gray@dshs.texas.gov |
| Microbiology Specimen Logistics | 512-776-3067 | micro.logistics@dshs.texas.gov |
| Newborn Screening Educators | 512-776-7585 | newbornscreeninglab@dshs.texas.gov |
| Newborn Screening Specimen Logistics | 512-776-3198 | dshsspecimenlogistics@dshs.texas.gov |
| Specimen Acquisition | (512) 776-7598 or 1-888-963-7111 ext. 7598 (toll free) |
We sincerely apologize for any inconvenience this interruption may cause. An update will be provided once the issue is resolved, and phone and fax services are back to normal operation.
The DSHS Austin Laboratory appreciates your patience and dedication to ensuring the best possible health outcomes for all Texans.
Posted October 14, 2024
Whole Genome Sequencing of Mycobacterium tuberculosis and Candida auris Now Available at the Texas DSHS Laboratory
The DSHS Austin Laboratory is now conducting whole genome sequencing (WGS) of submitted Mycobacterium tuberculosis and Candida auris specimens. Specimens selected for WGS are:
- confirmed M. tuberculosis obtained from clinical specimens and referred isolates.
- confirmed C. auris obtained from either colonization swabs or referred isolates.
Therefore, WGS may reflex for C. auris colonization testing and M. tuberculosis complex antimicrobial sensitivity test requests. No additional steps are required of submitters to order sequencing.
- Submitted M. tuberculosis complex specimens will be sequenced to better track the spread of antimicrobial resistant TB in Texas.
WGS is free for all M. tuberculosis and C. auris 
submitters in Texas. DSHS also provides specimen
collection swabs, mailers, and shipping for free.
NEW SUBMITTERS may set up a submitter account by downloading a Submitter ID Number Request Form, available on the DSHS Forms webpage at Laboratory Testing Services Manual - Forms and Laboratory Fee Schedule | Texas DSHS.
- Complete all applicable fields and email the form to labinfo@dshs.texas.gov or fax to 512-776-7533.
- A Lab Reporting team member will create a submitter account, provide a G-2B submission form, a supply order form, and Security Rights forms to request Web Portal access.
EXISTING SUBMITTERS may update their contact information by providing the updates in a Submitter ID Number Request Form and emailing the form to labinfo@dshs.texas.gov or faxing it to 512-776-7533.
Additional guidance on obtaining a DSHS submitter ID number, labeling specimens correctly, and completing submission forms is available on the DSHS Specimen Collection webpages.
SPECIMEN SUBMISSION QUESTIONS?
- Questions about C. auris and M. tuberculosis WGS: Email the Advanced Molecular Detection Group at WGS.DSHS@dshs.texas.gov, or call (512) 776-6614.
- Questions about submitting M. tuberculosis specimens: Email Mycobacteriology Team at (512) 776-7342, or email Mycobacteriology@dshs.texas.gov.
- Questions about submitting C. auris specimens: Email TexasARLN@dshs.texas.gov, or call (512) 776-7582.
Posted October 08, 2024
Correct Shipping and Delivery of Rabies Specimens to DSHS Austin Laboratory
DSHS Austin Laboratory – Microbiological Sciences Unit Notice
The DSHS Austin Laboratory reminds submitters that rabies specimens must be delivered directly to the Lab’s specimen receiving bay. The receiving bay is located on the north side of the Lab building, as shown in the map below:
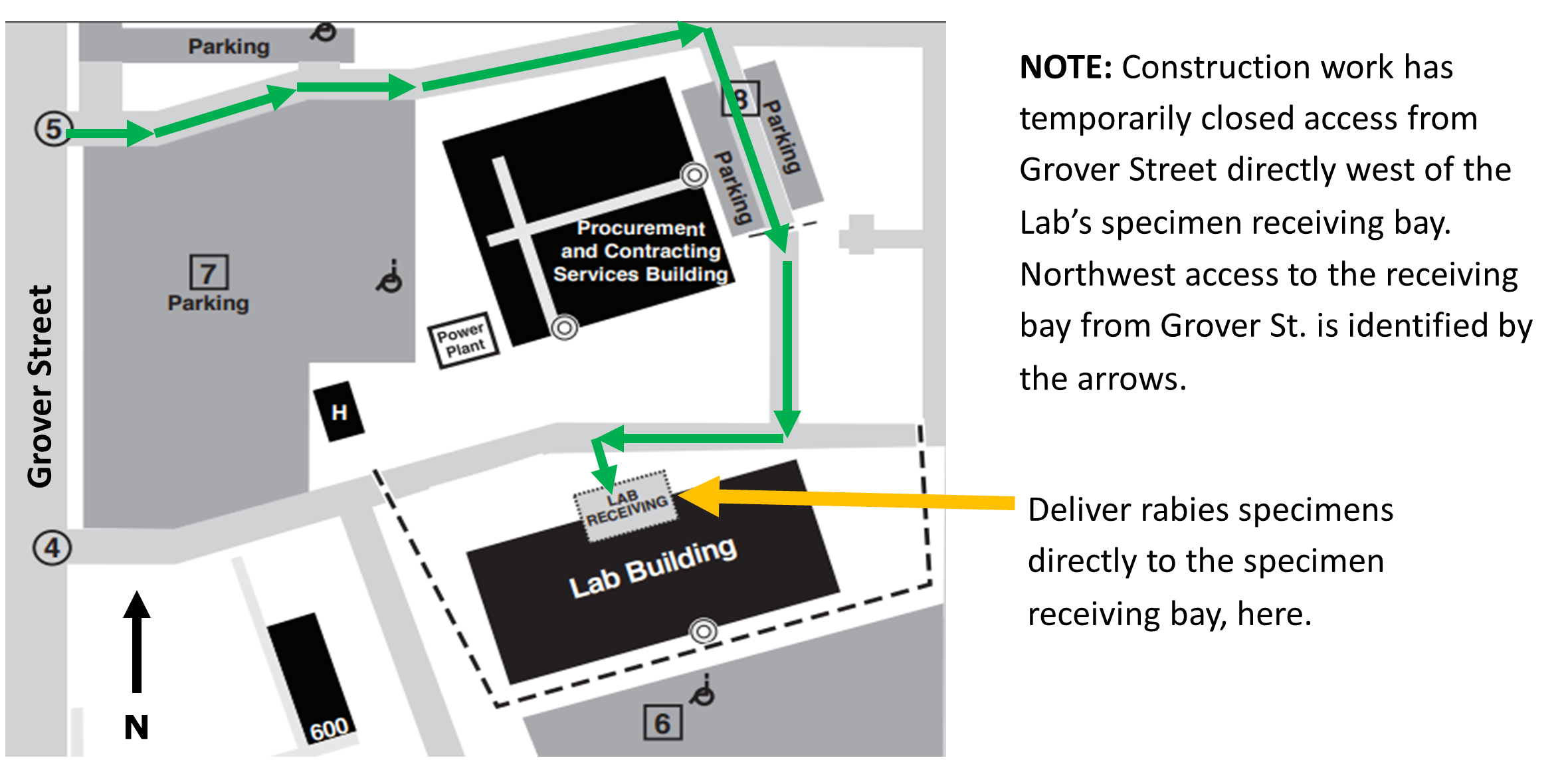
IMPORTANT
- Do not deliver rabies specimens to the Laboratory’s Security Desk or leave them by Lab entrance doors.
- Do not ship rabies specimens to the Laboratory via rideshare delivery apps.
- Rideshare services do not comply with US Department of Transportation (US DOT) requirements for the handling and shipping of infectious substances such as rabies specimens.
- Submitters may ship correctly labeled rabies specimens to the Lab via FedEx or UPS.
- Shipping specimens via USPS is discouraged.
Improper shipping and delivery of rabies specimens can potentially expose individuals to hazardous infectious substances and expose the submitter to significant legal penalties.
The DSHS Laboratory staff appreciate your attention to this matter.
Questions about Rabies Specimen Shipping?
DSHS Specimen Receiving
Call 512-776-7598 or 1-888-963-7111 ext. 7598 (toll free).
DSHS Rabies Team
Call 512-776-7595 or email rabies.team@dshs.texas.gov.
Overnight/Priority Shipping Address for Rabies Specimens
ATTN: Salvador Arreola
Texas Department of State Health Services
Public Health Laboratory Division
1100 W. 49th Street MC 1947
Austin, TX 78756-3199
Posted September 27, 2024
DSHS Austin Laboratory Phone and Fax Services Technical Issues - September 17, 2024
The DSHS Austin Laboratory is experiencing intermittent technical issues with outbound phone and fax systems and is unable to make phone calls or send faxes to various areas of Texas. The issue is being investigated and evaluated for resolution.
In the interim, the Laboratory is using alternative means to contact our submitters. If you receive a request to call the DSHS Laboratory, please do so. Alternatively, use email to initiate communication directly with your Laboratory contact.
| DSHS Austin Laboratory Contact | Phone Number | |
| AR Laboratory Network | TexasARLN@dshs.texas.gov | |
| AR Lab Network Liaison | 512-776-2321 | kimberly.abella@dshs.texas.gov |
| BioThreat Team | 512-776-3781 | dshslrn@dshs.texas.gov |
| Chemical Threat Team | 512-776-7270 | dshslrnc@dshs.texas.gov |
| Clinical Chemistry Educator | 512-776-6236 | jadyn.pena@dshs.texas.gov |
| Clinical Chemistry Specimen Logistics | 512-776-6237 or 512-776-2628 | clinicalchemistry@dshs.texas.gov |
| Container Supply/Kit Ordering | 512-776-7661 | ContainerPrepGroup@dshs.texas.gov |
| Environmental Chemistry Unit | 512-776-7587 | EnvSciAdmin@dshs.texas.gov |
| Laboratory Reporting Group | 512-776-7578 | labinfo@dshs.texas.gov |
| Microbiology Laboratory Educator | 512-776-3877 | niamh.gray@dshs.texas.gov |
| Microbiology Specimen Logistics | 512-776-3067 | micro.logistics@dshs.texas.gov |
| Newborn Screening Educators | 512-776-7585 | newbornscreeninglab@dshs.texas.gov |
| Newborn Screening Specimen Logistics | 512-776-3198 | dshsspecimenlogistics@dshs.texas.gov |
| Specimen Acquisition | (512) 776-7598 or 1-888-963-7111 ext. 7578 (toll free) |
We sincerely apologize for any inconvenience this interruption may cause. An update will be provided once the issue is resolved, and phone and fax services are back to normal operation.
The DSHS Austin Laboratory appreciates your patience and dedication to ensuring the best possible health outcomes for all Texans.
Posted September 17, 2024
Molecular Detection of Rickettsia SPP Now Available at the Texas DSHS Laboratory
Effective 8/26/2024, the Texas DSHS Austin Laboratory will begin accepting whole blood specimens collected in EDTA (purple/lavender top) tubes for the molecular detection and identification of Rickettsia spp. in symptomatic patients. This new Rickettsia polymerase chain reaction (PCR) test can detect and differentiate between Rickettsia rickettsii, the causative agent of Rocky Mountain Spotted Fever, and R. prowazekii, the causative agent of louse-borne or epidemic, typhus. The test also includes a pan-Rickettsia probe that can detect the presence of other rickettsial species, including R. typhi, the causative agent of flea-borne, also known as endemic or murine, typhus. However, it cannot identify R. typhi to the species level.
Per protocol, the following Rickettsia PCR test specimens will be forwarded to CDC by the DSHS Laboratory for confirmatory testing:
- Specimens that are presumptive positive for R. rickettsii or R. prowazekii.
- Specimens in which the pan-Rickettsia target is detected, but R. rickettsii and R. prowazekii are not.
- Indeterminate specimens approved by the CDC for further testing.
Note: Rickettsia spp. IgG antibody testing by indirect fluorescent antibody (IFA) is still offered at the Laboratory. We encourage providers to continue submitting serum specimens for this standard diagnostic test. Please refer to the DSHS Laboratory specimen and submission requirements for the IFA test at Rickettsia spp. Panel IgG.
The Rickettsia PCR test is expected to be added to the DSHS Laboratory Fee Schedule in early 2025. Until that time, there is no charge to submitters for this test.
The expansion of rickettsial disease testing at the Austin Laboratory will contribute to the surveillance and tracking of flea-borne typhus and spotted fever rickettsiosis, reportable conditions in Texas.
Questions?
- For questions about the new Rickettsia spp. PCR test at the Laboratory, please call the Clinical Virology team at 512-776-7594 or 512-776-2452 during regular business hours (Mon. – Fri. 8 a.m. to 5 p.m.), or email viral.isolation@dshs.texas.gov.
- For questions about the Rickettsia spp. IgG antibody test, please call the Serology Team at 512-776-7657 or 512-776-2505 during regular business hours, or email serological.analysis@dshs.texas.gov.
- Refer to the attached job aids for additional information on submitting rickettsial specimens to the DSHS Laboratory for Rickettsia testing.
- For more information on notifiable conditions in Texas, please visit https://www.dshs.texas.gov/notifiable-conditions.
- Please do not submit notifiable condition reports to the Laboratory!
Posted September 4, 2024
Becton, Dickinson and Company BACTECTM MGITTM 960 Pyrazinamide Test Kits Currently Unavailable
On July 25, 2024, the DSHS Laboratory in Austin received a letter from the CDC’s Division of TB Elimination (DTBE) indicating that phenotypic pyrazinamide (PZA) susceptibility testing kits may be unavailable to many laboratories due to a manufacturing issue at Becton, Dickinson and Company (BD). The list of affected test kit lots was expanded in an August 1st communication from BD, the manufacturer of the BACTECTM MGITTM 960 PZA test kits. It is currently unknown when new test kits will be available.
In response, the Texas DSHS Laboratory is collaborating with the CDC/DTBE, the Association of Public Health Laboratories (APHL), and several other public health laboratory partners to provide additional capacity for pncA gene sequencing. Sequencing of the pncA gene is an acceptable approach for determining PZA susceptibility as PZA resistance is primarily associated with pncA mutations.
In addition to providing services for the State of Texas’ public health customers, the DSHS Laboratory will be accepting Mycobacterium tuberculosis isolates from Arizona, Arkansas, Louisiana, Missouri, Tennessee, Virginia, and West Virginia. Please note that the CDC’s Molecular Detection of Drug Resistance (MDDR) service remains available to all jurisdictions for testing when drug resistance is suspected or known.
Demand for pncA gene mutation analysis may exceed the Lab’s pncA gene sequencing capacity. If you need pncA gene mutation analysis during this unavailability of phenotypic susceptibility testing, submitting labs should prioritize which isolates will be referred for pncA sequencing.
These might include isolates that are rifampin-resistant or multidrug-resistant (MDR) or isolates from patients whose treatment response is of concern.
- Note: Isolates evaluated by the CDC’s MDDR, or similar services, do not need to be resent for just pncA sequencing as this genetic locus is already included in the full panel for molecular testing.
For situations when PZA might be essential for building a multidrug treatment regimen, but PZA susceptibility cannot be ascertained due to either the lack of testing or the presence of a pncA mutation where correlation with PZA resistance is unknown, consultation with the Heartland National TB Center in San Antonio is strongly advised.
The DSHS Laboratory will provide updates as they become available.
Questions?
Call the DSHS Mycobacteriology Branch Manager at 512-776-7342 or email mycobacteriology@dshs.texas.gov
Posted August 23, 2024
DSHS Laboratory Antimicrobial Resistance Trends in Texas
The Texas Department of State Health Services (DSHS) Public Health Laboratory would like to extend our appreciation to all laboratorians, laboratory managers, and infection preventionists for your invaluable contributions to our Antimicrobial Resistance (AR) Laboratory. Your dedication and active participation in the national Antimicrobial Resistance Laboratory Network (ARLN) have been essential in advancing AR testing, raising public health awareness about AR threats, and enhancing our surveillance efforts.
We are excited to share insights into the types of organisms we have encountered in Texas and how these findings align with national public health trends. Read our AR Laboratory Clinical Engagement Letter, summarizing the key achievements of our AR laboratory.
Your continued engagement and expertise are crucial in addressing these emerging trends effectively. We look forward to our continued collaboration and working together to advance public health. Thank you once again for your commitment and support!
DSHS Laboratory Antimicrobial Resistance Website
Questions? Reach out to the AR Laboratory inbox TexasARLN@dshs.texas.gov
Posted August 16, 2024
DSHS Lab Web Application System Maintenance Scheduled Sunday, August 11, 9:00 a.m. to 11:00 a.m.
DSHS will be performing system maintenance 9:00 a.m. to 11:00 a.m. Sunday, August 11, 2024.
During this time, the DSHS Newborn Screening Web Application (Neometrics) and the DSHS Microbiology and Clinical Chemistry Web Application (Public Health Lab Online) may be unavailable.
Need help?
Contact DSHS Laboratory:
Phone: 1-888- 963-7111 ext. 7318 or Locally: 512-776-7318
Email: Labinfo@dshs.texas.gov
Posted August 9, 2024
DSHS Austin Laboratory Phone and Fax Services Technical Issues - July 9, 2024
The DSHS Austin Laboratory is experiencing intermittent technical issues with outbound phone and fax systems and is unable to make phone calls or send faxes to various areas of Texas. The issue is being investigated and evaluated for resolution.
In the interim, the Laboratory is using alternative means to contact our submitters. If you receive a request to call the DSHS Laboratory, please do so. Alternatively, use email to initiate communication directly with your Laboratory contact.
| DSHS Austin Laboratory Contact | Phone Number | |
| AR Laboratory Network | TexasARLN@dshs.texas.gov | |
| AR Lab Network Liaison | 512-776-2321 | kimberly.abella@dshs.texas.gov |
| BioThreat Team | 512-776-3781 | dshslrn@dshs.texas.gov |
| Chemical Threat Team | 512-776-7270 | dshslrnc@dshs.texas.gov |
| Clinical Chemistry Educator | 512-776-6236 | jadyn.pena@dshs.texas.gov |
| Clinical Chemistry Specimen Logistics | 512-776-6237 or 512-776-2628 | clinicalchemistry@dshs.texas.gov |
| Container Supply/Kit Ordering | 512-776-7661 | ContainerPrepGroup@dshs.texas.gov |
| Environmental Chemistry Unit | 512-776-7587 | EnvSciAdmin@dshs.texas.gov |
| Laboratory Reporting Group | 512-776-7578 | labinfo@dshs.texas.gov |
| Microbiology Laboratory Educator | 512-776-3877 | niamh.gray@dshs.texas.gov |
| Microbiology Specimen Logistics | 512-776-3067 | micro.logistics@dshs.texas.gov |
| Newborn Screening Educators | 512-776-7585 | newbornscreeninglab@dshs.texas.gov |
| Newborn Screening Specimen Logistics | 512-776-3198 | dshsspecimenlogistics@dshs.texas.gov |
| Specimen Acquisition | (512) 776-7598 or 1-888-963-7111 ext. 7578 (toll free) |
We sincerely apologize for any inconvenience this interruption may cause. An update will be provided once the issue is resolved, and phone and fax services are back to normal operation.
The DSHS Austin Laboratory appreciates your patience and dedication to ensuring the best possible health outcomes for all Texans.
Posted Jul 09, 2024
Ensure Rabies Specimen Mailers are Correctly Labeled for Shipping to DSHS Austin Laboratory
DSHS Austin Laboratory – Microbiological Sciences Unit Notice
The DSHS Austin Laboratory reminds submitters that to avoid shipping delays, mailing containers with rabies specimens MUST be labeled correctly.
The outer mailers of ALL rabies specimens must have
- the submitter’s name, street address, and contact phone number,
- a Biological Substance Category B, UN3373 label (sticker or hand-drawn),
- notices stating “ATTN: Rabies Team” and “Must be Refrigerated on Arrival”, and
- the name of the DSHS Laboratory’s Point of Contact: Salvador Arreola, the Laboratory’s Street address, and phone number.
Salvador Arreola,
Texas Department of State Health Services,
Public Health Laboratory Division,
1100 W. 49th Street,
Austin, TX 78756-3199
Phone: 512-776-7595

An example of how the outer mailer of a rabies specimen should be labeled.
If you do not have access to premade UN3373 stickers, you may draw the label directly onto the shipping box with a black permanent marker. The sides of the diamond must be at least 2 inches long. “Biological Substance Category B” must be written below the diamond.

All rabies specimens, including animal heads must be double bagged. Heads of small animals must be double bagged in sealable bags. Double bag the heads of larger animals in appropriately sized bags such as red biohazard bags or sturdy garbage bags. Bats MUST be deceased and in clear, sealable bags. Securely close all bags to prevent leaks.
NOTE: Ship specimens with ice packs to keep them cool in the elevated temperatures encountered during transit. High temperatures accelerate tissue decomposition which can compromise test results.
The Rabies Laboratory does not recommend shipping specimens via USPS. If there is no other shipping alternative available to you, please add the code “MC1947” to the Laboratory’s street address to ensure the specimen gets delivered correctly by USPS.
Refer to the printable guidance Submitting Rabies Specimens to the DSHS Laboratory for detailed packaging and shipping instructions.
Questions about Rabies Testing and Shipping?
Rabies Team: (512) 776-7595 or rabies.team@dshs.texas.gov
Specimen Acquisition Group: (512) 776-7598 or 1-888-963-7111
ext. 7598 (toll free)
Overnight/Priority Shipping Address for Rabies Specimens
Salvador Arreola
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7595
Posted Jul 3, 2024
Rabies Notification Form Available Online
The DSHS Austin Rabies Laboratory is pleased to announce the launch of the new rabies specimen notification form. The notification form can be completed and submitted online.
Submitters may still notify the Laboratory of a specimen submission by calling the Rabies Hotline at 1-800-252-8163.
Due to the serious nature of rabies, submitters are required by law to notify the Austin Laboratory that a rabies specimen is on its way. A G-9 specimen submission form is also required with each specimen. Completing and submitting a G-9 Form provides the Rabies Laboratory staff with vital information about potential human and/or animal exposure to rabies.
Remember!
- Do not submit live animals to the Laboratory.
- Texas summers are HOT. Ship specimens with multiple ice packs to keep them cold while in transit.
Notification + G-9 Form + Animal head (or whole bat/small rodent) = Testable Specimen
Refer to the printable guidance Submitting Rabies Specimens to the DSHS Laboratory for detailed packaging and shipping instructions.
Questions about Rabies Testing and Shipping?
Rabies Team: 512-776-7595 or rabies.team@dshs.texas.gov
Specimen Acquisition Group: 512-776-7598 or 1-888-963-7111
ext. 7598 (toll free)
Overnight/Priority Shipping Address for Rabies Specimens
Salvador Arreola
Texas Department of State Health Services
Public Health Laboratory Division
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7595
Posted Jul 3, 2024
DSHS Austin Laboratory Independence Day Closure
The DSHS Austin Microbiology Laboratory will be closed Thursday, July 4.
- Do not ship microbiology specimens for Thursday, July 4 delivery.
- Emergency rabies testing is available only for specimens dropped off before 8 a.m. Thursday, July 4.
- Emergency rabies testing requires prior authorization by 4:00 p.m. Wednesday, July 3 from the Rabies Laboratory.
- TB Elimination submitters should not collect or ship specimens on Wednesday, July 3. Due to the extreme heat, ice packs will not remain frozen over the holiday. Specimen quality may be affected, causing specimens to be rejected.
- Bacteriology water samples will not be accepted from Wednesday, July 3 to Friday, July 5.
- Regular water sample acceptance will resume Monday, July 8.
- Do not collect or ship gonorrhea antimicrobial susceptibility specimens Wednesday, July 3. These specimens must be tested within 24 hours of collection.
With the exception of bacteriology water sample analysis, the Microbiology Laboratory will resume normal business hours Friday, July 5.

SUMMER HEAT REMINDER: Please ship refrigerated specimens with multiple ice packs and frozen specimens with enough dry ice to keep them cold/frozen for up to 48 hours. Improve insulation and cushioning of specimen by packing empty space in shipping container with absorbent material.
Questions about Shipping and the Holiday Closure?
Rabies Team: (512) 776-7595 or rabies.team@dshs.texas.gov
TB Team: (512)-776-7342 or mycobacteriology@dshs.texas.gov
Specimen Intake Team: (512) 776-7598 or 1-888-963-7111 ext. 7598 (toll free)
Posted Jul 2, 2024
Specimens from Patients Under the Age of 14 are now Accepted for Gonorrhea and Chlamydia Screening at the DSHS Austin Laboratory
Effective 5/28/2024, the Texas Department of State Health Services (DSHS) Austin Laboratory is accepting specimens collected from patients under the age of 14 for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) screening by amplified RNA probe.
For information on the appropriate GC/CT screening specimen types and specimen submission details refer to the one-page flyer.
QUESTIONS?
Ordering kits: Email: ContainerPrepGroup@dshs.texas.gov
Specimen Collection/Suitability: Call (512) 776-7657 or 512-776-2449
Specimen Shipping: (512) 776-7598 or 1-888-963-7111 ext. 7578 (toll free)
Supply Ordering: (512) 776-7661 or ContainerPrepGroup@dshs.texas.gov
Submitter Accounts, Submission Forms, or Result Reports: (512) 776-7578 or LabInfo@dshs.texas.gov
Posted May 24, 2024
Malaria Testing at the DSHS Austin Laboratory
The DSHS Laboratory works with local and regional healthcare facilities and laboratories across Texas to rapidly detect parasitic diseases and prevent their spread. To ensure the timely detection of parasitic diseases, including suspected cases of malaria, the DSHS Laboratory reminds providers that testing for the presence of Plasmodium spp. in clinical specimens is available via Giemsa-stained morphological exam and Real-time Polymerase Chain Reaction (real-time PCR).
Required Specimens
- For Morphological Examination (by Giemsa Stain)
- Prepared Peripheral Blood Smears: Make thin and thick smears within one hour of specimen collection from finger prick collection.
- Whole Blood: Collect venipuncture blood in EDTA purple/lavender
top tubes.
- For Real-Time PCR Testing
- Whole Blood: Collect venipuncture blood in EDTA purple/lavender
top tubes.
- Whole Blood: Collect venipuncture blood in EDTA purple/lavender
Required Storage and Shipping Temperatures
- Thick and Thin Blood Smear Specimens:
- Ship at ambient temperatures but may also be submitted cold with whole blood specimens.
- Whole Blood Specimens:
- Store at 2°C–8°C. Ship overnight in insulated containers with cold packs.
Required Specimen Submission Form and Supplemental Information
- All specimens must be submitted with a G2-B Specimen Submission Form.
- Patient’s travel history and transfusion history is required.
NEW SUBMITTERS: To set up a submitter account, download a Submitter ID Number Request Form, available at Submitter ID Request February 2022 (texas.gov). Complete all applicable fields and email form to labinfo@dshs.texas.gov or fax to 512-776-7533. A Lab Reporting team member will create a submitter account, provide a G2-B submission form, a supply order form, and Security Rights forms to request Web Portal access.
Malaria Testing and Specimen Submission Questions?
For questions about morphological examination, real-time PCR testing or specimen suitability, please contact the Medical Parasitology Team at (512) 776-7560 or Medical.Parasitology@dshs.texas.gov.
Refer to the printable one-page flyers for more guidance on collecting and shipping malaria specimens to the DSHS Laboratory.
Posted May 15, 2024
Request for Submission of Cryptosporidiosis Specimens to Texas DSHS Laboratory
Cryptosporidium (Crypto) parasites cause the gastrointestinal illness cryptosporidiosis. Both the parasite and the illness are commonly called “Crypto.” Infection with Crypto can be life threatening to immunocompromised individuals. The Crypto parasites’ chlorine tolerance has led to it become a leading cause of recreational waterborne illness in the U.S. Outbreaks of Cryptosporidiosis have occurred every year in Texas for over 10 years.
The Texas Department of State Health Services (DSHS) is collaborating with the Centers for Disease Control and Prevention (CDC) in CryptoNet, a surveillance program developed to identify and track Cryptosporidium species, genotypes, and subtypes linked to illnesses.
We are requesting clinical laboratories send all appropriate* Cryptosporidium positive stool specimens to the DSHS Laboratory in Austin for molecular analysis. These specimen submissions will help improve the epidemiological understanding of cryptosporidiosis and improve the response to future outbreaks.
Why the Request to Submit Crypto Stool Specimens?
- Cryptosporidiosis is a Reportable Condition in Texas. Confirmed and suspected positive Crypto cases must be reported to the health department in the patient’s county of residence.
Submitting clinical specimens from these patients to the DSHS Laboratory is highly encouraged. - This Test is Free! Submitting laboratories will not be charged for testing specimens sent for this surveillance project. A final report with PCR results will be issued to submitters.
*For appropriate Crypto specimen type and specimen submission details, please refer to the one-page flyer.
NEW SUBMITTERS may set up a submitter account by downloading a Submitter ID Number Request Form, available on the DSHS website at Submitter ID Request (texas.gov). Complete all applicable fields and email the form to labinfo@dshs.texas.gov or fax to 512-776-7533. A Lab Reporting team member will create a submitter account, provide a G-2B submission form and Security Right forms to request Web Portal access.
EXISTING SUBMITTERS may update their contact information by providing the updates in a Submitter ID Number Request Form and emailing the form to labinfo@dshs.texas.gov or faxing 512-776-7533.
Additional guidance on obtaining a DSHS submitter ID number, labeling specimens correctly, and completing submission forms is available on the DSHS Specimen Collection webpages.
Shipping Information and Addresses
- Ensure Cary-Blair specimens are shipped cold.
- Ship specimens as a Category B Biological Substance, UN3373.
- Information on submitter responsibilities when shipping infectious substances is available on the DSHS Specimen Shipping webpages.
Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Public Health Laboratory Division
Department of State Health Services
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7569
Questions?
- For questions about Cryptosporidium testing or specimen suitability, please call the Parasitology Team at 512-776-7560 or email Medical.parasitology@dshs.texas.gov.
- For all other questions, please email FoodborneTexas@dshs.texas.gov.
Posted May 15, 2024
Request for Submission of Norovirus Specimens to the Texas DSHS Austin Laboratory
The Department of State Health Services (DSHS) Laboratory requests facilities submit norovirus (NoV) clinical specimens to the Laboratory for molecular analysis. Norovirus outbreaks tend to be underreported to public health officials, so the true incidence of this foodborne illness is likely higher. Testing patients for unknown gastrointestinal illnesses is essential for clinical decision-making and for community outbreak detection and investigations.
During outbreak investigations, your local or regional health departments may request that you forward clinical specimens to the DSHS Laboratory for molecular analysis. It is essential that you submit requested specimens to help DSHS epidemiologists link individual cases during outbreak investigations.
FREE NoV TEST - There is no charge to the submitter for testing clinical specimens requested for NoV outbreak investigation purposes.
Please Note: Testing is free only for NoV specimens that are requested by an epidemiologist for outbreak investigations.
SHIP NoV Specimens COLD - Specimens that arrive ambient cannot be tested. Be sure to ship with enough cold packs to keep the specimens cool for up to 48 hours.
Refer to the printable one-page flyers for more guidance on collecting and shipping norovirus specimens to the Laboratory.
NEW SUBMITTERS may set up a submitter account by downloading a Submitter ID Number Request Form, available on the DSHS website at Submitter ID Request (texas.gov). Complete all applicable fields and email the form to labinfo@dshs.texas.gov or fax to 512-776-7533.
A Lab Reporting team member will create a submitter account, provide a G-2B submission form, and Security Right forms to request Web Portal access.
EXISTING SUBMITTERS may update their contact information by providing the updates in a Submitter ID Number Request Form and emailing the form to labinfo@dshs.texas.gov or faxing 512-776-7533.
Shipping Address for Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Public Health Laboratory Division,
Department of State Health Services
1100 W. 49th Street
Austin, TX 78756-3199
Phone: 512-776-7569
Norovirus Questions?
- For laboratory questions about NoV testing or specimen suitability, please contact the Molecular Biology Team Lead at (512) 776-6510.
- For questions related to DSHS’ NoV surveillance project (CaliciNet), please email FoodborneTexas@dshs.texas.gov.
For more information on specimen packaging and shipping, check out the shipping and submission guidance webpage.
Posted May 14, 2024
Request for Submission of Cyclospora Specimens to Texas DSHS Austin Laboratory
Cyclosporiasis is a foodborne disease caused by the protozoan parasite Cyclospora cayetanensis. Most cyclosporiasis cases tend to occur seasonally between the months of April and August. Symptoms of cyclosporiasis include abdominal pain and severe, watery diarrhea that can last several weeks. Immunocompromised individuals are at increased risk of severe complications from the illness.
The Texas Department of State Health Services (DSHS) is collaborating with the Centers for Disease Control and Prevention (CDC) to identify and sequence Cyclospora specimens linked to cyclosporiasis outbreaks. Therefore, we request clinical laboratories send all appropriate* Cyclospora positive stool specimens to the DSHS Austin Laboratory for molecular analysis.
These specimens will help us detect disease clusters more quickly and improve the public health response to future outbreaks.
Why the Request to Submit Cyclospora Stool Specimens?
- Cyclosporiasis is a Reportable Condition in Texas. Confirmed and suspected positive cyclosporiasis cases must be reported to the health department in the patient’s county of residence. Submitting clinical specimens from these patients to the DSHS Lab is highly encouraged.
- This Test is Free! Submitting providers or laboratories will not be charged for testing and sequencing specimens submitted for this surveillance project. A final report with PCR results will be sent to submitters.
*For appropriate Cyclospora specimen type and specimen submission details, refer to the informational flyer.
NEW SUBMITTERS: To set up a submitter account, download a Submitter ID Number Request Form. Complete all applicable fields and email to labinfo@dshs.texas.gov or fax to 512-776-7533. A Lab Reporting team member will create a submitter account, provide a G-2B submission form, a supply order form, and Security Rights forms to request Web Portal access.
Additional guidance on obtaining a DSHS submitter ID number, labeling specimens correctly, and complete submission forms is available on the DSHS Specimen Collection webpages.
Shipping Information and Addresses
Ship specimens overnight as a Category B Biological Substance, UN3373. Information on submitter responsibilities when shipping infectious substances is available on the DSHS Specimen Shipping webpages.
Overnight/Priority Courier Service
Specimen Receiving: Walter Douglass
Laboratory Services Section
Department of State Health Services
1100 W. 49th Street
Austin, TX 78756-3199
Questions?
- Cyclospora Testing or Specimen Suitability: Contact the Medical Parasitology Team at (512) 776-7560 or email Medical.parasitology@dshs.texas.gov.
- All Other Questions: Email FoodborneTexas@dshs.texas.gov
Posted April 12, 2024
A message from Grace Kubin, Ph.D., Associate Commissioner, DSHS Public Health Laboratory Division
It’s that time of year again! Each year, the DSHS Austin Public Health Laboratory conducts a survey, open to all our customers, to provide important feedback on laboratory services. Your feedback is used by the laboratory to better understand your needs and identify gaps in service as part of the laboratory's commitment to continuous quality improvement. If you are interested to view the results of last year’s survey: 2023 Annual DSHS Laboratory Customer Service Survey Results Summary.
I encourage you to take the 2024 Public Health Laboratory Customer Service Survey. It should not take more than 10 minutes to complete. For questions or concerns, please send an email to: Labinfo@dshs.texas.gov.
2024 Public Health Laboratory Customer Service Survey
**Feedback will be collected until April 30, 2024**
Posted April 1, 2024
DSHS Austin Laboratory Thanksgiving Holiday Closure Reminder
With the exception of Newborn Screening and Rabies testing, the DSHS Austin Laboratory will be closed, and no testing will occur Thursday, 11/23/2023 through Saturday, 11/25/2023. The DSHS Laboratory is completely closed on Sundays. Testing services will resume as normal, Monday, 11/27/2023.
See below for more details on selected testing.
Newborn Screening (NBS)
- The Newborn Screening Laboratory will be closed Thanksgiving Day, Thursday 11/23/2023 and will resume testing on Friday, 11/24/2023.
- DO NOT DELAY collection or shipment of newborn screening specimens.
- Ship dried specimens within 24 hours of collection. If mail or courier services are unavailable, ship as soon as possible.
- DSHS recommends shipping specimens using an overnight or trackable service like USPS priority mail, FedEx, and UPS.
Clinical Chemistry
- Total hemoglobin, lead, glucose, and lipid/cholesterol specimens must be received by Wednesday, 11/22/2023 to complete testing before the holiday closure.
- DO NOT ship cholesterol, lipid profile, or glucose specimens on Fridays or on the day before a state-observed holiday.
Microbiology
Rabies Specimens:
- Rabies specimens received after 8:00 a.m. Wednesday, 11/22/2023 will be tested Friday, 11/24/2023. Submitters will be notified of positive results by 12:00 p.m. Friday. All other results will be available Monday 11/27/2023.
- Emergency testing will be available Friday, 11/24/2023 and Saturday, 11/25/2023 only by prior arrangement with Rabies testing staff. Call (512) 776-7595 before 4:00 p.m. Wednesday, 11/22/2023 to discuss. Specimen receipt must be guaranteed by 8:00 a.m. the morning of testing. Preliminary results will be called out as soon as emergency testing is completed. Final results will be available Monday 11/27/2023.
- All other specimens will be tested on Monday, 11/27/2023, and results will be available by 5:00 p.m.
Tuberculosis (TB) Specimens:
- Do not ship specimens for delivery between Wednesday, 11/22/2023 and Saturday, 11/25/2023. Refrigerate specimens collected during this period until shipping is possible.
- Ensure critical AFB Smear and NAA for M. tuberculosis specimens are delivered by 8:30 a.m. Wednesday, 11/22/2023.
- Call (512) 776-7342 before 9:00 a.m. on Wednesday, 11/22/2023 for expedited test requests.
Candida auris Swab Specimens:
- Do not ship swabs for delivery from Wednesday, 11/22/2023 to Saturday, 11/24/2023.
Swabs collected during this period must be refrigerated and shipped on cold packs for delivery when Laboratory is open.
Carbapenem Resistant Organisms Swab Specimens:
- Do not collect or ship swabs from Tuesday, 11/21/2023 to Saturday, 11/24/2023.
Neisseria gonorrhoeae Antimicrobial Susceptibility Testing Specimens:
- Do not collect or ship swabs from Tuesday, 11/21/2023 to Saturday, 11/24/2023.
Bacteriology Water Samples:
- Water samples will not be accepted at DSHS Laboratory from Wednesday, 11/22/2023 to Saturday, 11/25/2023.
All Other Microbiology Specimens or Samples:
- Do not ship specimens for delivery between Thursday, 11/23/2023 and Saturday, 11/25/2023. Store specimens collected during this time at an appropriate temperature, e.g. -70°C, or below for clinical virology samples.
- No tests will be performed from Thursday, 11/23/2023 to Saturday, 11/25/2023.
Ordering Laboratory Supplies:
Newborn Screening Medicaid/Paid Card Order Requests
- Priority overnight shipping requests received on Wednesday 11/22/2023 will be processed and shipped via FedEx Priority Overnight.
- All requests received before noon Wednesday 11/22/2023 will be processed and shipped via FedEx Ground.
- Requests received after noon Wednesday 11/22/2023 through Saturday 11/25/2023 will be sent Monday, 11/27/2023.
Texas Health Steps/PKU/TB/HIV/Courier Supply Order Requests
- All requests received on Wednesday 11/22/2023 will be audited, issued, and shipped via FedEx Ground.
- Requests received before 9:00 a.m. on Wednesday 11/22/2023 will be audited, issued, and shipped via FedEx Ground Wednesday afternoon, 11/22/23.
- Requests received after 9:00 a.m. on Wednesday 11/22/2023 through Sunday 11/26/2023 will be audited, issued, and shipped on Monday 11/27/2023 via FedEx Ground.
***Per FedEx website, FedEx Ground will be closed Thursday 11/23/2023 but will deliver packages Friday 11/24/2023.***
Questions?
Newborn Screening General Inquiries: (512) 776-7333
Microbiology General Inquiries: (512) 776-7599
Clinical Chemistry General Inquiries: (512) 776-6236
Newborn Screening: NewbornScreeningLab@dshs.texas.gov
Clinical Chemistry: ClinicalChemistry@dshs.texas.gov
Microbiology Branch: Lab.Microbiology@dshs.texas.gov
Specimen Submission: LabInfo@dshs.texas.gov
Container Preparation: ContainerPrepGroup@dshs.texas.gov
Posted November 9, 2023
DSHS Laboratory – Microbiological Sciences Unit Notice
Effective November 1, 2023, the Texas DSHS Austin Laboratory will no longer provide serologic testing services for Plague (Yersinia pestis), IgG.
If specimens for this assay are received at the Laboratory on or after November 1, 2023, Laboratory staff will contact the submitter to choose how to proceed with the specimens from the options below:
- Option 1: Cancel the test and appropriately discard the specimens.
- Option 2: Forward the specimens to Centers for Disease Control and Prevention (CDC) for testing with no shipping charges for submitter. Yersinia pestis serology testing at CDC has a projected turnaround time of two weeks.
Yersinia pestis Serology Testing Questions?
Contact the Serological Analysis Group
Phone: (512) 776-7760 or 1 (888) 963-7111 ext. 7760 (Toll Free)
Email: Lab.Microbiology@dshs.texas.gov
Posted Nov 01, 2023
Retirement of Bordetella spp. PCR and Culture Testing Services at DSHS Laboratory
DSHS Laboratory – Microbiology Unit Notice
Retirement of Bordetella spp. Real-Time Polymerase Chain Reaction and B. pertussis Culture Identification Testing Services at DSHS Laboratory
Effective October 1, 2023, the DSHS Austin Microbiology Laboratory will no longer conduct Bordetella spp. Real-Time PCR and B. pertussis culture identification testing services.
Questions?
- For questions about Bordetella spp. testing, please contact the Molecular Biology Team at (512) 776-7784 or email Lab.Microbiology@dshs.texas.gov
Posted Sep 27, 2023
****Effective Immediately****
Measles IgM Testing Information
Due to delays in the manufacture of Measles IgM EIA kits, the DSHS Laboratory is temporarily unable to perform Measles IgM testing. During this time, all specimens received at DSHS Laboratory requesting this test will be forwarded to Quest Diagnostics laboratory for processing.
Measles IgM test results will continue to be reported from DSHS Laboratory but will include an additional attached report displaying the Quest results.
Please continue to submit specimens for Measles IgM testing to DSHS Laboratory following specimen submission requirements.
DSHS Laboratory anticipates the Measles IgM EIA kits will be available again no later than March 2024. The Laboratory will release a follow-up notification when the issue is resolved.
Questions or Comments?
Call toll-free: 1-888-963-7111 ext. 7760
Call Local: 512-776-7760
Email: Lab.Microbiology@dshs.texas.gov
Posted Sep 21, 2023
Do Not Submit Live Animals to DSHS Laboratory for Rabies Testing
The DSHS Austin Laboratory reminds submitters that we cannot accept live animals for rabies testing. Please refrain from delivering or shipping live bats or other live animals to the Laboratory.
Bats must be submitted
- deceased,
- in a clear, sealable bag, **New Requirement**
- triple packed, per submission guidelines.
Double bag the heads of larger animals in appropriately sized bags such as red biohazard bags or sturdy garbage bags. Securely close the bags to prevent leaks.
NOTE: Ship all specimens with ice packs to keep them cool in the elevated temperatures encountered during transit. High temperatures accelerate tissue decomposition which can compromise rabies test results.
Refer to the printable guidance Submitting Rabies Specimens to the DSHS Laboratory for detailed packaging and shipping instructions.
Questions about Rabies Testing and Shipping?
- Rabies Team: (512) 776-7595 or rabies.team@dshs.texas.gov
- Specimen Acquisition Group: (512) 776-7598 or 1-888-963-7111 ext. 7598 (toll free)
Overnight/Priority Shipping Address
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756-3199
Posted Aug 11, 2023
Upcoming Training Webinars
During September and November 2022, the Denver Prevention Training Center and Texas Department of State Health Services will host two webinars on the management of gonorrhea and syphilis in Texas.
Managing Superbugs: Antibiotic Resistant Gonorrhea (GC) in Texas
Topics to be discussed include:
- Current trends in the prevalence of gonorrhea and antibiotic resistant N. gonorrhoeae in Texas.
- The new GC test to be offered by DSHS—gonorrhea antimicrobial susceptibility testing.
Date: Thursday, Sept. 29, 2022
Time: 12:00 p.m.–2:00 p.m. CST
Register: https://courses.nnptc.org/class_information.html?id=3422
Continuing Education Credits: 2 hours (CME/NCPD)
Cost: Free
Exploring an Epidemic: Managing Syphilis During Pregnancy in Texas
Topics to be discussed include:
- Current trends in the prevalence of syphilis in the general population and in pregnant women in Texas.
- Statewide efforts to address congenital syphilis.
Date: Wednesday, Nov. 2, 2022
Time: 12:00 p.m.–2:00 p.m. CST
Registration
Continuing Education Credits: 2 hours (CME/NCPD)
Cost: Free
Posted September 20, 2022
Effective 8/22/2022, the Texas Department of State Health Services Laboratory is now providing initial serologic screening testing services for chronic Chagas disease (Chagas IgG).
Medical providers are encouraged to submit serologic specimens to the DSHS Laboratory for screening. Chagas disease, which is caused by infection with the protozoan parasite Trypanosoma cruzi, is a reportable condition to DSHS. The expansion of Chagas disease testing services at the Austin Laboratory will contribute to the surveillance and tracking of this reportable disease in Texas.
View a description of the Chagas IgG tests, acceptable specimen types, and possible test results.
For questions about Chagas testing or specimen suitability, contact the Serological Analysis Team at 512-776-7657 or 512-776-7760.
For information regarding specimen packaging and shipping, call Specimen Receiving at (512) 776-7598.
Posted September 16, 2022
Beat the Summer Heat: Tips on How to Package Temperature-Sensitive Specimens to Keep Them Cold in Transit
DSHS Austin Laboratory – Microbiological Sciences and Clinical Chemistry Branch Notice
The DSHS Austin Laboratory reminds submitters to pack temperature-sensitive specimens with enough refrigerant to keep them frozen or cold for up to 48 hours to account for the hot Texas summers and elevated temperatures in transport vehicles.
Several specimen types, including whole blood, plasma, serum, stool, exudate, rabies, and several clinical and environmental swabs specimens require cold shipping to the laboratory. Specimens that need to be shipped cold but arrive at the lab at ambient temperature are deemed to be unacceptable and may not be testable.
Tips on packing and shipping temperature-sensitive specimens:
- Ship temperature sensitive specimens overnight to the DSHS laboratory as soon as possible after collection.
- Identify the appropriate refrigerant for the specimen (e.g., dry ice, frozen gel packs, ice packs, or wet ice).
- Pack specimen with enough refrigerant to keep it cold for up to 48 hours to account for the possibility of delays in delivery to the lab, particularly during the hot Texas summer temperatures
- Pack remaining gaps in the container with additional absorbent insulation (such as newspaper, paper towels, cotton wadding, etc.) to reduce the volume and movement of warm air within the container. Minimizing open space in containers will keep contents colder for longer.
- Do NOT ship for a weekend or holiday delivery; specimens are accepted during regular business hours only* (Mon. – Fri. 8:00 a.m. – 5:00 p.m.). Alternatively, keep specimen chilled until it can be delivered on a weekday.
*Saturday delivery of rabies specimens for same day testing is available in emergency situations only. Specimens must arrive at lab before 8:00 a.m. for same-day results. The rabies lab must first be notified by phone or email about a specimen submission. Call the Rabies Notification line at 1-800-252-8163 (toll free) or complete the Electronic Rabies Specimen Submission Notification Form.
The DSHS Laboratory staff thank you for your assistance with optimizing specimen submission and testing processes!
Additional Submission and Shipping Guidance Is Available
Antibiotic Resistance Lab Network Refer to specimen preparation and submission guidelines for carbapenem resistant organisms and Candida spp.
Arbovirus Specimen Submitters: Refer to specimen packaging and shipping guidance at Arbovirus Field Surveillance Techniques.
Clinical Specimen Submitters: Refer to the appropriate test description in the LTSM’s Microbiology Test list for shipping and specimen handling requirements.
Food Sample Submitters: Refer to the food sample packaging and shipping instructions on the Consumer Microbiology Lab’s webpage.
Rabies Specimen Submitters: Refer to specimen handling and shipping guidance on the Rabies Lab’s webpage.
Questions about Shipping or Specimen Packaging/Container?
Call Specimen Receiving at (512) 776-7598
Laboratory Shipping Address
For Overnight/Priority Shipping
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756-3199
Posted August 4, 2022
Closure of Austin Greyhound Bus Station: Alternative Means of Submitting Rabies and Mosquito Specimens to Texas DSHS Austin Laboratory Required
The Department of State Health Services (DSHS) Austin Laboratory has been notified that the Greyhound Bus terminal at 916 E Koenig Ln. Austin, TX 78751 will permanently close May 31, 2022, but are ceasing freight 15, 2022. The Austin terminal closure will affect the ground transportation options available for shipping rabies and mosquito specimens to the Laboratory. From May 15 onward, rabies specimens will need to be delivered directly to the Austin Laboratory or transported by an alternative courier service of your choice. DSHS is currently investigating alternative shipping arrangements and will notify submitters of new options, if available. Should you have questions about the closure of the Greyhound station, call (512) 776-7598.
Note: Specimens sent to the Austin Greyhound terminal for pick up by laboratory staff on or after May 15 cannot not be collected and could be returned or lost!
- Packages transported by ground and overnight courier services (USPS, FedEx, UPS, LoneStar Overnight, etc.) are not affected by the Austin Greyhound terminal closure and will be accepted at the Laboratory during regular business hours Monday–Friday (8:00 a.m.–5:00 p.m.).
- Hand-delivered samples are unaffected by the closure and may be delivered to Specimen Acquisition (Laboratory Specimen Receiving Dock) during regular business hours. Refer to the DSHS campus map for direction to the Specimen Receiving Dock, which is highlighted in yellow.
- Regulatory packaging and shipping requirements are unaffected by the closure. Per regulations 49 CFR § 171-173, all rabies specimens must be packaged and properly labeled for shipping as a Biological Substance, Category B UN3373. Mosquito specimens must be shipped using the plastic collection cartons and insulated boxes provided by the Laboratory.
- Submitters of rabies specimens are still required to call the toll-free Rabies Hotline at 1-800-252-8163 to alert the Laboratory of the expected arrival time and to provide any other relevant information about the shipment.
- DSHS does not recommended specimens be shipped for weekend or holiday delivery, including by couriers that may offer weekend deliveries.
In the Event of an Emergency Situation
- Rabies Specimens: For emergency processing of a specimen on a Saturday, the submitter must first receive prior approval from the DSHS Laboratory. Contact the Rabies Identification Team before 4 p.m. on Friday at (512) 776-7595. After 4 p.m. Friday, call (512) 776-7111 and request to speak with a regional Zoonosis Control staff member. The specimen must arrive at the lab by 8 a.m. Saturday.
- Mosquito Specimens: Call the DSHS Arbovirus/Entomology Laboratory Team at (512) 776-2442, or toll free at
(888) 963-7111 ext. 2442 to discuss special testing requests.
Additional Shipping and Packaging Information
Shipping Addresses
Rabies Specimens
ATTN: Rabies Team
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756
Be sure to label the package with BIOLOGICAL SUBSTANCE, CATEGORY B UN3373, and REFRIGERATE ON ARRIVAL
For laboratory questions about rabies testing or specimen suitability, contact the Rabies Identification Team at
(512) 776-7595, or email nachea.qualls@dshs.texas.gov.
Mosquito Specimens
ATTN: Arbovirus/Entomology Team
Texas Department of State Health Services
Laboratory Services Section
1100 W. 49th Street
Austin, TX 78756
For laboratory questions about mosquito/arbovirus testing or specimen suitability, contact Bethany Bolling at (512) 776-2442, or email bethany.bolling@dshs.texas.gov.
Posted May 6, 2022